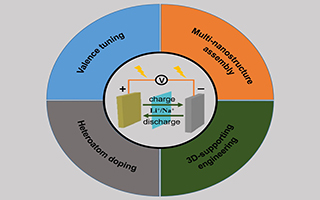
发展低成本、高性能、高安全的锂离子、钠离子电池是解决能源储存问题的一个重要途径. 由于具有丰富的化学价态,开放式的化学结构和较高的理论容量,钒基材料是一种非常有潜力的锂离子电池、钠离子电池电极材料. 在过去的几年中,钒基电极材料如钒的氧化物、硫化物、磷酸盐等在电池中的应用取得了长足的进展,有必要对相关的研究进展作一个总结. 本文介绍了钒基电极材料的近期研究进展,重点总结了钒基电极材料应用所面临的离子扩散系数低、结构稳定性差等科学问题,并从活性材料本身的改性以及与外部材料复合作用两个角度重点分析了应对这些问题所采用的策略. 一方面,通过对钒元素的化合价态进行调控来提高材料的电导性,并采用异原子掺杂来加快离子扩散系数. 另一方面,借助同/异种纳米结构间的耦合作用增强材料的结构稳定性. 基于基底的骨架作用,实现三维有序阵列结构电极的制备,进而促进材料能量密度与功率密度的共同提升. 最后,讨论了钒基材料进一步发展所面临的挑战,希望能够为将来相关电极材料的研究提供一些参考.
It is an important solution to solve energy storage problems by developing inexpensive and safe lithium-ion and sodium-ion batteries with superior performance. Vanadium-based electrode materials are promising electrode materials because of diversified chemical valences, open structures and high theoretical capacities. In the past few years, vanadium-based electrode materials such as oxides, sulfides, and phosphates have achieved a considerable development in the battery field, It is, therefore, necessary to summarize their recent research progress. In this review, we particularly highlight the key challenges that are facing in the application of vanadium materials, such as low ion diffusion coefficient and poor structural stability. The possible solutions that are capable of addressing these challenges are analyzed from modification of active materials and their interaction with other materials. On the one hand, the enhancement of conductivity and ion diffusivity can be realized by valence tuning and heteroatoms doping, respectively. In addition, hierarchical assembly maximizes structural stability of materials during cycles, and 3D array engineering enable the improvement of power and energy density of battery simultaneously. We hope that this review can provide insights into the further development of vanadium-based electrode materials.