

基于电化学方法测定饮用水源水中的痕量铜离子
收稿日期: 2018-04-18
修回日期: 2018-05-02
网络出版日期: 2019-12-28
基金资助
国家自然科学基金项目(No. 21473140)、福建省产学研合作项目(No. 2016Y4012)和中央高校基本科研业务费专项资金(No. 20720160113)资助
Electrochemical Determination of Trace Copper ions in Drinking Water Source
Received date: 2018-04-18
Revised date: 2018-05-02
Online published: 2019-12-28
Supported by
This work was financially supported by the Natural Science Foundation of China (No. 21473140), the Collaboration Project between Industry and University in Fujian Province (No. 2016Y4012), and the Fundamental Research Funds for the Central Universities (No. 2072016011).
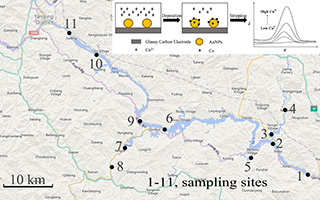
彭劲骥 , 郑 红 , 邹义松 , 刘国坤 , 袁东星 . 基于电化学方法测定饮用水源水中的痕量铜离子[J]. 电化学, 2019 , 25(6) : 699 -707 . DOI: 10.13208/j.electrochem.180418

/
| 〈 |
|
〉 |