

锂离子电池高镍正极材料掺铝的作用及掺铝方法的研究
收稿日期: 2018-03-28
修回日期: 2018-04-23
网络出版日期: 2019-12-28
基金资助
福建省科技重大专项 (No. 2014HZ0002-1)、福建省科技创新领军人才项目资助
Effects of Al-Doping on Properties of Ni-Rich Cathode Materials Employing Different Aluminum Sources
Received date: 2018-03-28
Revised date: 2018-04-23
Online published: 2019-12-28
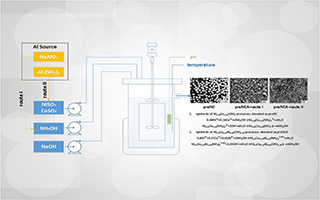
王丹凤 , 李益孝 , 王伟立 , 杨 勇 . 锂离子电池高镍正极材料掺铝的作用及掺铝方法的研究[J]. 电化学, 2019 , 25(6) : 660 -668 . DOI: 10.13208/j.electrochem.180328

/
| 〈 |
|
〉 |