

双二苯基-1-甲基咪唑膦氯化镍的制备及其电催化还原CO2的研究
收稿日期: 2017-04-04
修回日期: 2017-05-09
网络出版日期: 2017-05-11
基金资助
国家自然科学青年基金项目(No. 21303053 )资助
Preparation of Ni(dpim)2Cl2 Complex for the Electrocatalytic Reduction of CO2
Received date: 2017-04-04
Revised date: 2017-05-09
Online published: 2017-05-11
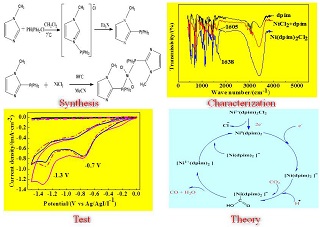
以二苯基-1-甲基咪唑膦(dpim)为配体制备了一种新型的配合物催化剂Ni(dpim)2Cl2. 循环伏安研究表明,Ni(dpim)2Cl2配合物在氮气气氛下表现出两步还原的电化学行为,在-0.7 V下为两电子的不可逆还原,在-1.3 V下为单电子准可逆还原. 向电解液中通入CO2后,在-1.3 V下的还原峰变得不可逆,且其峰电流从0.48 mA·cm-2增大到0.55 mA·cm-2. 在质子源(CH3OH)存在的条件下,该还原峰电流可继续增大到0.72 mA·cm-2. 该研究结果表明,Ni(dpim)2Cl2配合物对CO2还原具有良好的电催化性能,且其电催化还原过程符合ECE机理. 在-1.3 V下恒电位电解得到的还原产物主要为CO,催化转换频率(Turnover of Frenquency, TOF)为0.17 s-1.
关键词: 双二苯基-1-甲基咪唑膦氯化镍; 甲醇; 二氧化碳; 电催化
张历朴 , 钮东方 , 张新胜 . 双二苯基-1-甲基咪唑膦氯化镍的制备及其电催化还原CO2的研究[J]. 电化学, 2018 , 24(2) : 103 -110 . DOI: 10.13208/j.electrochem.170401
A new Ni(dpim)2Cl2complex using 2-(diphenylphosphino)-1-methylimidazole (dpim) as ligand was prepared and served as a catalyst for the electrochemical reduction of CO2. The electrochemical redox behavior of Ni(dpim)2Cl2 in CH3CN/TPABF4 solution under nitrogen atmosphere showed the two-electron irreversible reduction at -0.7 V and one-electron quasi-reversible reduction at -1.3 V. After bubbling CO2 into the electrolyte, the reduction peak appeared at -1.3 V became irreversible and the peak current increased from 0.48 mA·cm-2 to 0.55 mA·cm-2. Moreover, the peak current at -1.3 V could further increase to 0.72 mA·cm-2 in the presence of proton source (CH3OH). These observations indicate that the Ni(dpim)2Cl2 exhibited a good electrocatalytic performance toward CO2 reduction and the electrocatalytic reduction process followed the ECE mechanism. The reduction products obtained by the potentiostatic electrolysis (at -1.3 V) were identified to be mainly CO with the catalytic conversion frequency of 0.17 s-1.

Key words: Ni(dpim)2Cl2; methanol; CO2; electrocatalysis
/
| 〈 |
|
〉 |