

不同羧酸对钒改性硅酸铁锂结构性能的影响
收稿日期: 2017-03-16
修回日期: 2017-05-05
网络出版日期: 2017-05-12
基金资助
获国家自然科学基金(No. 11372263)资助
Effects of Carboxylic Acids on Structure and Performance of 10% Vanadium Modified Li2FeSiO4/C Composites
Received date: 2017-03-16
Revised date: 2017-05-05
Online published: 2017-05-12
Supported by
Supported by The National Natural Science Foundation of China (No. 11372263)
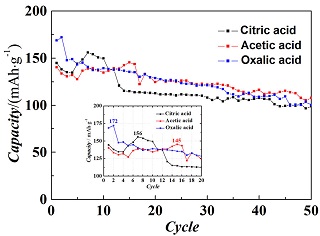
采用溶胶-凝胶法得到前驱体,再通过固相烧结法制备10%钒改性碳包覆硅酸铁锂正极材料,系统考察了三种羧酸(即柠檬酸、乙酸、草酸)添加剂对10%钒改性碳包覆硅酸铁锂正极材料的相结构、表面形貌、界面特性和电化学性能的影响. 结果表明,三种羧酸添加剂制备的材料都能得到结晶性较好的P21和Pmn21混合相结构,主要杂质相为铁,此外还存在少量偏硅酸锂杂质. 以柠檬酸、乙酸、草酸为添加剂合成的10%钒改性碳包覆硅酸铁锂在0.1C倍率下的首次放电容量分别为144.7、140.3和168.7 mAh•g-1,最大容量分别为155.9、145.3和172.0 mAh•g-1出现在第7、15和2周,经50周循环后容量保持率分别为68.2%、76.7%和59.4%. 柠檬酸单位分子内含有三个羧酸根,以柠檬酸为添加剂合成的材料残留碳含量最高为7.8%,促进了铁杂质的形成,较大的电荷传质电阻(147 Ω)使得库伦效率较低,循环性能较差。相反,乙酸分子中只含有一个羧酸根,以乙酸为添加剂合成的材料中铁杂质相最少,电荷传质电阻(73 Ω) 最低,导致容量保持率最高,循环性能最好. 草酸分子中含有两个羧酸根,以草酸为添加剂合成的材料形成较大的花状形貌,得到合适的残留碳含量(6%),极大地提高了锂离子迁移率(3.85×10-15 cm2•s-1),从而取得超过一个锂离子(1.05)的脱嵌.
魏雪霞 , 黄嘉祺 , 刘施阳 , 程璇 , 张颖 . 不同羧酸对钒改性硅酸铁锂结构性能的影响[J]. 电化学, 2018 , 24(1) : 72 -80 . DOI: 10.13208/j.electrochem.170316
The carbon coated 10% vanadium modified lithium iron silicate (Li2Fe0.9V0.1SiO4/C) composites were prepared by sol-gel method to form precursor and followed by solid state reaction. Effects of different carboxylic acids, namely, citric acid, acetic acid and oxalic acid, on the crystal structures, surface morphologies, interfacial characteristics and electrochemical properties of the composites were systematically investigated. It was found that a mixed P21 and Pmn21 phase was formed with the major impure phase of iron (Fe) and minor impurity of lithium silicate (Li2SiO3). The initial discharge capacities of 144.7, 140.3 and 168.7 mAh•g-1 were achieved at 0.1C and room temperature, while the maximum capacities of 155.9, 145.3 and 172.0 mAh•g-1 at the 7th, 15th and 2nd cycles with the capacity retention values of 68.2%, 76.7% and 59.4% were obtained upon 50 cycles for the uses of citric acid, acetic acid and oxalic acid, respectively. Consisting of three carboxyl functional groups, the citric acid based composite contained higher amount of 7.8% residual carbon, the formation of impure Fe phase was promoted, and the larger charge-transfer resistance of 147 Ω was obtained, leading to lower coulombic efficiency and poorer cycle performance. On the contrast, the acetic acid based composite containedone carboxyl functional group only, resulted in the least amount of Fe and the smaller charge-transfer resistance of 73 Ω ,which showed the best cycle performance with the largest capacity retention. However, carrying two carboxyl functional groups the oxalic acid based composite led to 6.0% residual carbon and larger flower-like morphology, which slightly improved the lithium ion diffusion coefficient, achieving more than one lithium ion (1.05) per formula unit intercalation.

/
| 〈 |
|
〉 |