

高镍三元正极材料动力学性能的单颗粒研究
收稿日期: 2017-02-07
修回日期: 2017-03-19
网络出版日期: 2017-04-20
基金资助
宁德时代新能源科技股份有限公司项目(No. RD-RXNPRFTM001)资助
Intrinsic Kinetic Properties of Ternary Material for Lithium Ion Batteries Assessed by Single Particle Microelectrode
Received date: 2017-02-07
Revised date: 2017-03-19
Online published: 2017-04-20
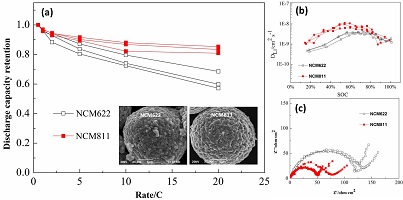
镍钴锰三元材料LiNixCoyMnzO2 (x + y + z = 1)在容量、倍率、循环及热稳定性等方面的性能往往受到金属元素Ni、Co、Mn含量的显著影响. 其中,增加元素Ni的含量有助于提高材料的比容量。因此,LiNi0.6Co0.2Mn 0.2O2(NCM622)和LiNi0.8Co0.1Mn0.1O2(NCM811)成为了目前研究最为广泛的两款高镍三元正极材料. 但目前针对这两款材料的对比研究主要集中在材料比容量、热稳定性和循环稳定性的影响方面,而对材料动力学性能的研究较少,尤其是对材料本征动力学参数的表征尚未见报道. 本文采用单颗粒微电极技术,以粒径相同的 NCM622 和 NCM811 颗粒为研究对象,排除导电剂、粘结剂和电极结构的影响,从材料本征动力学性能评估的角度出发,分析了Ni元素的含量对这两款材料的充放电性能、交流阻抗谱、锂离子固相扩散系数和倍率放电性能等的影响. 结果表明,与 NCM622 相比,随着Ni2+和Ni3+总含量的增加,NCM811 表现出更高的充放电容量、锂离子固相扩散系数、电化学反应活性和倍率放电性能. 以 20 C 放电, NCM811 材料的放电容量保持率仍可达到80.8%以上.
魏奕民 . 高镍三元正极材料动力学性能的单颗粒研究[J]. 电化学, 2018 , 24(1) : 81 -88 . DOI: 10.13208/j.electrochem.170209
Electrochemical performances such as capacity, rate, cycle and thermal stability of the nickel (Ni), cobalt (Co) and manganese (Mn) ternary cathode material, LiNixCoyMnzO2 (x + y + z = 1), are significantly influenced by the proportion of Ni, Co, and Mn elements. To obtain higher specific capacity, LiNi0.6Co0.2Mn 0.2O2 (NCM622) and LiNi0.8Co0.1Mn0.1O2 (NCM811) with high amounts of Ni element were employed for the lithium ion batteries. By now, many studies have been focusing on the thermal and cycling stabilities of NCM622 and NCM811. However, there is lack of reports on the intrinsic kinetic properties of these two cathode materials. In this work, single particle microelectrode has been employed to investigate the intrinsic kinetic properties of NCM622 and NCM811 without the influences of binder, conductive agent, and electrode structure. Charge-discharge test, electrochemical impedance spectroscopy (EIS), and potentiostatic intermittent titration (PITT) methods were used for the evaluation of the kinetic properties of NCM622 and NCM811. Due to the increased Ni2+/Ni3+ and decreased Mn4+ amounts, the NCM811 material presented better kinetics properties and higher columbic efficiency compared with NCM622. The discharge capacity retention of NCM811 was above 80.8% at 20C compared to 0.5C, which is much higher than that of NCM622 with 68.6%.

/
| 〈 |
|
〉 |