

乙腈/碳酸氢钠溶液混合物电嫁接叔丁氧羟基-乙二胺
收稿日期: 2016-12-01
修回日期: 2017-01-21
网络出版日期: 2017-01-29
基金资助
H. Hamzah would like to thank the Majlis Amanah Rakyat Malaysia (MARA) for the PhD scholarship. P. Bartlett gratefully acknowledges receipt of a Wolfson Research Merit award.
Electrografting of Mono-N-Boc-Ethylenediamine from an Acetonitrile/Aqueous NaHCO3 Mixture
Received date: 2016-12-01
Revised date: 2017-01-21
Online published: 2017-01-29
Supported by
H. Hamzah would like to thank the Majlis Amanah Rakyat Malaysia (MARA) for the PhD scholarship. P. Bartlett gratefully acknowledges receipt of a Wolfson Research Merit award.
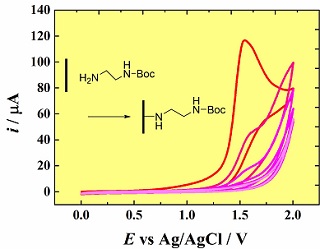
碳电极表面的伯胺电嫁接被广泛地应用于电极表面改性. 本文通过比较发现,在比例为4:1的乙腈和0.1 mol•L-1 碳酸氢钠溶液混合物中,玻碳电极表面嫁接上叔丁氧羟基-乙二胺的效率比在单纯的乙腈中显著提高. 有碳氢酸钠存在时,循环伏安测得电极的初始电流增大,表明电极表面胺嫁接层的形成更快速,从而使得电极表面更容易钝化,导致乙二胺-叔丁氧羟基膜层更严重地阻碍[Fe(CN)6]3-. 的反应. 通过去除叔丁氧羟基保护层,在蒽醌-2-羧酸上接自由胺,可获得较高的蒽醌表面覆盖度. 采用简单动力学模型模拟了电嫁接反应,结果表明,模拟得到的循环伏安曲线与实验测得的循环伏安曲线相一致. 对比在单纯乙腈和乙腈/碳酸氢钠溶液混合物中模型拟合得到的参数值可知,胺自由基与碳表面的反应和在均相溶液中的反应相互竞争,更有利于乙腈与碳氢酸钠的表面反应.
Hisham Hamzah , Guy Denuault , Philip Bartlett , Aleksandra Pinczewska , Jeremy Kilburn . 乙腈/碳酸氢钠溶液混合物电嫁接叔丁氧羟基-乙二胺[J]. 电化学, 2017 , 23(2) : 130 -140 . DOI: 10.13208/j.electrochem.161241
The electrografting of primary amines to carbon electrodes is now widely employed for electrode modification. Using a mixture of acetonitrile and 0.1 mol•L-1 aqueous sodium hydrogen carborate (NaHCO3) in the ratio of 4:1, the efficiency for coupling of mono-N-Boc-ethylenediamine (EDA-Boc) on the surface of glassy carbon was significantly improved as compared with that obtained using acetonitrile alone. In the presence of NaHCO3 the initial current determined in the cyclic voltammogram became higher, and the layer of attached amine was formed more rapidly, accordingly, the electrode was passivated more rapidly. The resulting film of EDA-Boc was shown to be more severely blocking toward the electrochemical reaction of [Fe(CN)6]3-. Following removal of the Boc protecting group and coupling of the free amine to anthraquinone-2-carboxylic acid, a higher surface coverage of the anthraquinone was obtained. Modelling for the electrograftng reaction using a simple kinetic scheme, it was demonstrated that the simulated voltammograms agreed well with the experimentally measured voltammograms . Comparison between the model fitting parameters obtained from the acetonitrile alone and the acetonitrile/NaHCO3 mixture showed that the competition between reaction of the amine radicals with the carbon surface and reaction in the homogeneous solution became more favourable for the surface reaction in the acetonitrile/NaHCO3 mixture.

[1] Adenier A, Chehimi M M, Gallardo I, et al. Electrochemical oxidation of aliphatic amines and their attachment to carbon and metal surfaces[J]. Langmuir, 2004, 20(19): 8243-8253.
[2] Barbier B, Pinson J, Desarmot G, et al. Electrochemical bonding of amines to carbon fiber surfaces toward improved carbon-epoxy composites[J]. Journal of the Electrochemical Society, 1990, 137(6): 1757-1764.
[3] Downard A J. Electrochemically assisted covalent modification of carbon electrodes[J]. Electroanalysis, 2000, 12(14): 1085-1096.
[4] Bélanger D, Pinson J. Electrografting: a powerful method for surface modification[J]. Chemical Society Reviews, 2011, 40(7): 3995-4048.
[5] Deinhammer R S, Ho M, Anderegg J W, et al. Electrochemical oxidation of amine-containing compounds: a route to the surface modification of glassy carbon electrodes[J]. Langmuir, 1994, 10(4): 1306-1313.
[6] Liu J, Dong S. Grafting of diaminoalkane on glassy carbon surface and its functionalization[J]. Electrochemistry Communications, 2000, 2(10): 707-712.
[7] Holm A H, Vase K H, Winther-Jensen B, et al. Evaluation of various strategies to formation of pH responsive hydroquinone-terminated films on carbon electrodes[J]. Electrochimica Acta, 2007, 53(4): 1680-1688.
[8] Buriez O, Labbé E, Pigeon P, et al. Electrochemical attachment of a conjugated amino–ferrocifen complex onto carbon and metal surfaces[J]. Journal of Electroanalytical Chemistry, 2008, 619–620(0): 169-175.
[9] Buriez O, Podvorica F I, Galtayries A, et al. Surface grafting of a π-conjugated amino-ferrocifen drug[J]. Journal of Electroanalytical Chemistry, 2013, 699(0): 21-27.
[10] Tanaka M, Sawaguchi T, Sato Y, et al. Surface modification of GC and HOPG with diazonium, amine, azide, and olefin derivatives[J]. Langmuir, 2011, 27(1): 170-178.
[11] Geneste F, Moinet C. Electrochemically linking TEMPO to carbon via amine bridges[J]. New Journal of Chemistry, 2005, 29(2): 269-771.
[12] Nasraoui R, Bergamini J-F, Ababou-Girard S, et al. Sequential anodic oxidations of aliphatic amines in aqueous medium on pyrolyzed photoresist film surfaces for the covalent immobilization of cyclam derivatives[J]. Journal of Solid State Electrochemistry, 2011, 15(1): 139-146.
[13] Chrétien J-M, Ghanem M A, Bartlett P N, et al. Covalent tethering of organic functionality to the surface of glassy carbon electrodes by using electrochemical and solid-phase synthesis methodologies[J]. Chemistry a European Journal, 2008, 14(8): 2548–2556.
[14] Chrétien J-M, Ghanem M A, Bartlett P N, et al. Covalent modification of glassy carbon surfaces by using electrochemical and solid-phase synthetic methodologies: application to bi- and trifunctionalisation with different redox centres[J]. Chemistry a European Journal, 2009, 15(44): 11928-11936.
[15] Ghanem M A, Chrétien J-M, Kilburn J D, et al. Electrochemical and solid-phase synthetic modification of glassy carbon electrodes with dihydroxybenzene compounds and the electrocatalytic oxidation of NADH[J]. Bioelectrochemistry. 2009, 76(1-2): 115-125.
[16] Ghanem M A, Chrétien J-M, Pinczewska A, et al. Covalent modification of glassy carbon surface with organic redox probes through diamine linkers using electrochemical and solid-phase synthesis methodologies[J]. Journal of Materials Chemistry, 2008, 18(41): 4917-4927.
[17] Sosna M, Chretien J-M, Kilburn J D, et al. Monolayer anthracene and anthraquinone modified electrodes as platforms for Trametes hirsuta laccase immobilisation[J]. Physical Chemistry Chemical Physics. 2010, 12(34): 10018-10026.
[18] Pinczewska A, Sosna M, Bloodworth S, et al. High-throughput synthesis and electrochemical screening of a library of modified electrodes for NADH oxidation[J]. Journal of the American Chemical Society, 2012, 134(43): 18022-18033.
[19] Groppi J, Bartlett P N, Kilburn J D. Toward the control of the creation of mixed monolayers on glassy carbon surfaces by amine oxidation[J]. Chemistry a European Journal, 2016, 22(3): 1030-1036.
[20] Baranton S, Bélanger D. Electrochemical derivatization of carbon surface by reduction of in situ generated diazonium cations[J]. Journal of Physical Chemistry B, 2005, 109(51): 24401-24410.
[21] Saby C, Ortiz B, Champagne G Y, et al. Electrochemical modification of glassy carbon electrode using aromatic diazonium salts .1. Blocking effect of 4-nitrophenyl and 4-carboxyphenyl groups[J]. Langmuir, 1997, 13(25): 6805-6813.
[22] Ghanem M A, Kocak I, Al-Mayouf A, et al. Covalent modification of carbon nanotubes with anthraquinone by electrochemical grafting and solid phase synthesis[J]. Electrochimica Acta. 2012, 68: 74-80.
[23] Bhugun I, Savéant J M. Derivatization of surfaces and self-inhibition in irreversible electrochemical reactions - cyclic voltammetry and preparative-scale electrolysis[J]. Journal of the Electrochemical Society, 1995, 395: 127-131.
[24] Allongue P, Delamar M, Desbat B, et al. Covalent modification of carbon surfaces by aryl radicals generated from the electrochemical reduction of diazonium salts[J]. Journal of the American Chemical Society, 1997, 119(1): 201-207.
/
| 〈 |
|
〉 |