

壳层厚度可调控的Ag@Pd@Pt纳米粒子的合成和甲酸电催化研究
收稿日期: 2016-09-23
修回日期: 2016-11-28
网络出版日期: 2016-12-02
基金资助
国家自然科学基金项目(No. 2011YQ030124)资助
Syntheses of Ag@Pd@Pt Nanoparticles with Tunable Shell Thickness for Electrochemical Oxidation of Formic Acid
Received date: 2016-09-23
Revised date: 2016-11-28
Online published: 2016-12-02
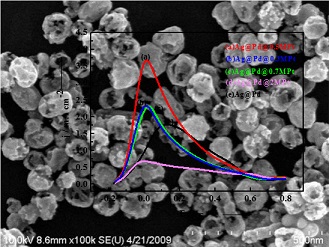
在本课题组研究55 nm Au@Pd@Pt对甲酸电催化效果基础上,我们采用Ag取代Au制备55 nm Ag@Pd@Pt纳米粒子以降低催化剂的成本,并对甲酸的电催化行为进行研究. 研究表明:少量Pt的存在可大幅度提高催化剂的活性,当Pt的覆盖度为0.5 单原子层(ML)时,起始氧化电位最为靠前,氧化峰电流最大,这与Au@Pd@Pt纳米粒子对甲酸电催化行为类似. 与Au@Pd@Pt纳米粒子相比,其最佳起始氧化电位偏正0.05 V,但电催化活性并没有明显的降低. 通过改变催化剂比表面积研究甲酸的电催化行为,发现将9 nm Ag纳米粒子作为内核的9 nm Ag@Pd@Pt负载在活性炭中,在保持催化活性不变的情况下,碳载的催化剂价格可比55 nm Au@Pd@Pt纳米粒子降低220倍左右.
林晓东 , 陈杜宏 , 田中群 . 壳层厚度可调控的Ag@Pd@Pt纳米粒子的合成和甲酸电催化研究[J]. 电化学, 2016 , 22(6) : 570 -576 . DOI: 10.13208/j.electrochem.160569

/
| 〈 |
|
〉 |