

超级电容器用凹凸棒石负载氮掺杂碳@NiCo2O4复合电极材料的制备及其电化学性能研究
Preparation and Electrochemical Properties of Attapulgite-Supported Nitrogen-Doped Carbon@NiCo2O4Composites for Supercapacitors
Received date: 2016-05-23
Revised date: 2016-07-05
Online published: 2016-07-29
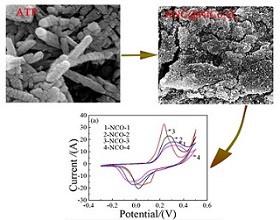
通过在凹凸棒石表面原位聚合苯胺制得聚苯胺包覆凹凸棒石,经高温热处理得到凹凸棒石(ATP)负载氮掺杂碳(ANC),然后通过水热-煅烧法在ANC表面负载NiCo2O4制得ANC@NiCo2O4复合材料. 采用FTIR、XRD、SEM、TEM和BET表征其化学组成和微观结构,通过恒流充放电(GCD)和循环伏安法(CV)测试其电化学性能.结果表明,ANC较高的比表面积和疏松多孔的形貌,使水热NiCo2O4颗粒能够均匀分散在其表面,与电解液的接触面积较大,赋予复合材料良好的电化学性能. 复合材料在1 A·g-1时质量比电容可达945.5 F·g-1,16 A·g-1时质量比电容为587.6 F·g-1,保持率为62.1%,表现出较好的倍率特性.在12 A·g-1大电流下循环充放电2000次后,质量比电容保持率达74.1%,高于水热纯纳米NiCo2O4的48.7%,表明ANC@NiCo2O4复合材料具有较好的循环稳定性.
万慧 , 应宗荣 , 刘信东 , 卢建建 , 张文文 . 超级电容器用凹凸棒石负载氮掺杂碳@NiCo2O4复合电极材料的制备及其电化学性能研究[J]. 电化学, 2017 , 23(1) : 28 -35 . DOI: 10.13208/j.electrochem.160523
In this work, the attapulgite-supported nitrogen-doped carbon (ANC) was prepared by in-situ chemically polymerizing polyaniline coating upon attapulgite, followed by high temperature heat treatment, and then NiCo2O4was reacted onto the surface of ANC by a combination of hydrothermal reaction and calcination to synthesize ANC@NiCo2O4 composites. The chemical composition and morphology of the samples were characterized by Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM) and N2 adsorption/desorption. The electrochemical properties were evaluated by means of constant current charge discharge (GCD) and cyclic voltammetry (CV). The results showed that due to the high specific surface area and porous structure of ANC, the NiCo2O4 particles were uniformly located on the surface, resulting in large contact reaction areas with the electrolyte and improved electrochemical performance. At a current density of 1 A·g-1, the specific capacitance was up to 945.5 F·g-1, while at a current density of 16 A·g-1, it was 587.6 F·g-1, i.e., the capacitance retention was 62.1%, revealing a better rate performance. After 2000 cycles of charge-discharge process at a high current of 12 A·g-1, the capacitance retention was 74.1%, higher than 48.7% of pure NiCo2O4, which indicated that the as-prepared composite electrode materials had excellent electrochemical durability.

Key words: Attapulgite; Carbon; Nitrogen-doped; NiCo2O4; Electrode material; Supercapacitor
/
| 〈 |
|
〉 |