

负载型离子液体在C-H键电氧化活化中的应用研究
收稿日期: 2016-03-12
修回日期: 2016-04-12
网络出版日期: 2016-05-18
基金资助
973前期研究专项(No. 2012CB722604)资助
Application of Composite Ionic Liquid in Electro-oxidation Activation of a C-H Bond
Received date: 2016-03-12
Revised date: 2016-04-12
Online published: 2016-05-18
本文将1-乙基-3-甲基咪唑醋酸盐离子液体修饰在多壁碳纳米管上,制备出离子液体/碳纳米管复合材料,并研究了对甲氧基甲苯(p-MT)在该复合离子液体水溶液体系中的电氧化性能. 同时,通过循环伏安法和计时电流法考查了扫描速率、温度、反应底物浓度等因素对电氧化性能的影响,研究了p-MT在该体系中的动力学过程. 实验结果表明,p-MT在复合离子液体水溶液体系中发生不可逆的电氧化反应,且该过程受扩散控制,扩散系数为7.69×10-10 cm2·s-1. 适当地升高温度和增大反应底物浓度都有利于促进p-MT中C-H键选择性电氧化为相应醛基,选择性可达到95%. 通过在不同结构电解槽中进行恒电位电解研究,发现离子液体/MWCNTs复合电解质在一室型电解槽中进行p-MT电氧化的电解效率更高、对目标产物对甲氧基苯甲醛(p-MBA)的选择性也更好.
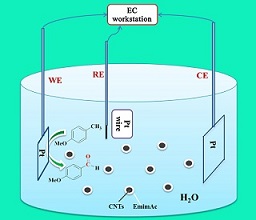
关键词: C-H键氧化; 离子液体/碳纳米管复合材料; 电氧化活化; 对甲氧基苯甲醛
陈姿颖 , 武倩倩 , 张健青 , 朱英红 , 马淳安 . 负载型离子液体在C-H键电氧化活化中的应用研究[J]. 电化学, 2017 , 23(1) : 1 -6 . DOI: 10.13208/j.electrochem.160315
In this paper, the ionic liquid/carbon nanotube composite material was prepared through modifying the ionic liquid 1-ethyl-3-methylimidazolium acetate to multi-walled carbon nanotubes. The electro-oxidation properties of p-methoxy toluene (p-MT) were studied using the composite as an electrolyte. The effects of scanning speed, temperature and substrate concentration were studied by cyclic voltammetry and chronoamperometry. The electrochemical kinetics of p-MT in this system was also studied. The results showed that the electrochemical oxidation of p-MT in the composite electrolyte solution was irreversible. The process was mainly controlled by diffusion, and the diffusion coefficient (D) was 7.69×10-10 cm2.s-1. Increasing the reaction temperature and the concentration of substrate could promote the electro-oxidation of the C-H bond to the corresponding aldehyde, and the selectivity of p-methoxybenzaldehyde (p-MBA) could improve to 95%. The electrolysis in one-pot was more efficiency and achieved better selectivity than that in the H-type.

/
| 〈 |
|
〉 |