

多尺度炭材料担载铂作为稳定的电催化剂用于氧还原反应
收稿日期: 2016-01-04
修回日期: 2016-04-15
网络出版日期: 2016-04-28
基金资助
This work is financially supported by the Strategic Priority Research Program of the Chinese Academy of Science (Grant No. XDA09030104), the National Basic Research Program of China (973 Program, Grant No. 2012CB215500) and the Key Program of the Chinese Academy of Science (Grant No. KGZD-EW-T08).
Multi-Scaled Carbon Supported Platinum as a Stable Electrocatalyst for Oxygen Reduction Reaction
Received date: 2016-01-04
Revised date: 2016-04-15
Online published: 2016-04-28
Supported by
This work is financially supported by the Strategic Priority Research Program of the Chinese Academy of Science (Grant No. XDA09030104), the National Basic Research Program of China (973 Program, Grant No. 2012CB215500) and the Key Program of the Chinese Academy of Science (Grant No. KGZD-EW-T08).
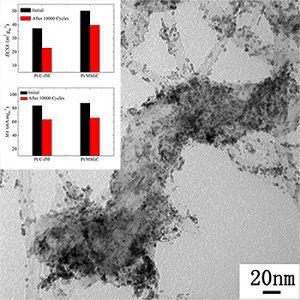
炭载体的稳定性对于燃料电池电催化剂是至关重要的. 本文中采用酚醛树脂作为前驱体,二氧化硅为模板剂,制备了多介孔且石墨化程度高的炭载体(HGMC). 相比于商品Vulcan XC-72,HGMC具有中等的比表面积和高的石墨化程度,因此在电位循环扫描过程中具有较高的化学稳定性,然而HGMC碳层堆叠的结构不利于传质. 为克服这一劣势,多壁碳纳米管(MWCNTs)作为隔离物加入至HGMC中以构建具有三维多尺度结构的载体(MSGC). 与HGMC为载体担载Pt以及商品催化剂Pt/C-JM相比,由于炭载体的具有高稳定性以及三维多尺度结构,MSGC担载Pt后不仅使电催化剂的电化学稳定性提高,且氧还原反应过程中传质得到显著改善.
李玉萍 , 姜鲁华 , 王素力 , 孙公权 . 多尺度炭材料担载铂作为稳定的电催化剂用于氧还原反应[J]. 电化学, 2016 , 22(2) : 135 -146 . DOI: 10.13208/j.electrochem.151149
The stability of carbon supports is essential for electrocatalysts of fuel cells. Herein, the mesoporous carbon with the high graphitic degree (highly graphitic mesoporous carbon, HGMC) was synthesized by using resorcinol as the carbon precursor, SiO2 as the templates and urea as the reducing agent. The as-prepared HGMC was characterized by XRD, Raman spectroscopy, TEM, N2 adsorption. The stability of HGMC was evaluated by mimicking the start-up/shut-down conditions of fuel cells in a three-electrode system referring to the NREL standard. The obtained HGMC is of moderate surface area (187.4 m2•g-1) and is chemically stable under potentiodynamic cycling as compared to the commercial Vulcan XC-72, while the high graphitic structure is adverse to the mass transport. To overcome the drawback of the HGMC in mass transportation, MWCNTs was introduced as a spacer to construct a 3D multi-scaled support. Compared to the single HGMC supported Pt catalyst and the commercial Pt/C-JM catalyst, the multi-scaled MSGC (the mixture of HGMC and MWCNTs with a mass ratio of 1:1) supported Pt catalyst displayed both enhanced electrochemical stability and significantly improved mass transportation for the oxygen reduction reaction, due to the stability and the multi-scaled structure of the carbon supports.

Key words: carbon support; platinum; oxygen reduction reaction; fuel cell
/
| 〈 |
|
〉 |