

柠檬酸中锌防蚀剂(1,3-Dioxolan-2-ylmethyl)三苯基溴化磷和碘离子的协同效应研究
收稿日期: 2015-12-22
修回日期: 2016-03-10
网络出版日期: 2016-04-14
基金资助
The author wishes to thank Prof. Dr. Beshir Ahmed Abd El-Nabey, Prof. Dr. Essam Khamis and Prof. Dr. Ashraf Moustafa, Department of chemistry, Faculty of Science, Alexandria University, Alexandria, Egypt for continuous support and providing advanced instruments.
An investigation of the synergistic effect between (1,3-Dioxolan-2-ylmethyl)–triphenylphosphonium bromide and iodide ion for the corrosion inhibition of zinc in citric acid
Received date: 2015-12-22
Revised date: 2016-03-10
Online published: 2016-04-14
Supported by
The author wishes to thank Prof. Dr. Beshir Ahmed Abd El-Nabey, Prof. Dr. Essam Khamis and Prof. Dr. Ashraf Moustafa, Department of chemistry, Faculty of Science, Alexandria University, Alexandria, Egypt for continuous support and providing advanced instruments.
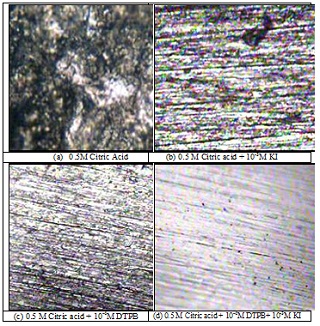
关键词: zinc; polarization; EIS; acid corrosion; synergism
M. Saadawy . 柠檬酸中锌防蚀剂(1,3-Dioxolan-2-ylmethyl)三苯基溴化磷和碘离子的协同效应研究[J]. 电化学, 2017 , 23(4) : 441 -455 . DOI: 10.13208/j.electrochem.151222

Key words: zinc; polarization; EIS; acid corrosion; synergism
/
| 〈 |
|
〉 |