

玻碳电极表面因瓦合金的电化学成核机理
收稿日期: 2016-03-18
修回日期: 2016-03-29
网络出版日期: 2016-04-05
基金资助
国家自然科学基金项目(21321062)资助
Electrochemical Nucleation of Invar Alloy on Glassy Carbon Electrode
Received date: 2016-03-18
Revised date: 2016-03-29
Online published: 2016-04-05
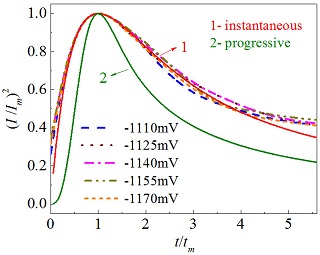
在弱酸性因瓦合金(含镍质量分数为32~36 % 的镍铁合金)镀液中, 以线性扫描伏安法、循环伏安法和恒电位阶跃法对因瓦合金在玻碳电极表面的电沉积过程及其成核机理进行研究. 结果表明, 在该体系下, 因瓦合金在玻碳电极表面的电结晶属于扩散控制下的不可逆电极过程. 运用Scharifker-Hills理论模型(SH)拟合实验数据表明, 因瓦合金在玻碳电极表面的共沉积更加符合三维瞬时成核的成核规律. 运用Heerman-Tarallo理论模型(HT)分析得到因瓦合金在玻碳电极表面的成核生长的动力学参数, 当阶跃电位从-1.11 V变化至-1.17 V (vs SCE), 成核密度数(N0)由0.72×105 cm-2提高至1.91×105 cm-2, 成核速率常数(A)从 40.35 s-1增至 194.38 s-1, 扩散系数(D)为(7.67±0.15)×10-5 cm2•s-1, 变化不大.
黄先杰 , 闫慧 , 黄帅帅 , 杨防祖 , 田中群 , 周绍民 . 玻碳电极表面因瓦合金的电化学成核机理[J]. 电化学, 2017 , 23(1) : 7 -12 . DOI: 10.13208/j.electrochem.160318
Abstract: The linear sweep voltammetry, cyclic voltammetry and potential step methods were used to study the electrodeposition mechanism of Invar nickel-iron alloy (the mass fraction of nickel was 32~36%) on glassy carbon electrode surface in the weak acidic bath. The results demonstrate that the electrodeposition is was a diffusion controlled irreversible electrode process in this system. The Scharifker-Hill (SH) theory theoritic model (SH) were was used employed to fitting the experimental data and the result shows that the codeposition of Invar alloy on glassy carbon electrode surface conformed to the diffusion controlled three-dimensional instantaneous nucleation mechanism. The kinetic parameters were obtained by the Heerman-Tarallo (HT) theory theoretic model (HT). When the step potential shifted from -1.11 V to -1.17 V, the active nucleation sites density (N0) increased from 0.72×105 cm-2 to 1.91 ×105 cm-2. The nucleation rate constant (A) raised from 40.35 s-1 to 194.38 s-1 and the diffusion coefficient (D) was(7.67±0.15)×10-5 cm2•s-1, remaining basically constant.

/
| 〈 |
|
〉 |