

LiF-BaF2-LiCl熔盐体系中Li+的电化学行为
收稿日期: 2015-09-21
修回日期: 2015-10-11
网络出版日期: 2015-11-19
基金资助
国家自然科学基金项目(No. 3203204516)资助
The Electrochemical Behavior of Li+ in LiF-BaF2-LiCl Molten Salt System
Received date: 2015-09-21
Revised date: 2015-10-11
Online published: 2015-11-19
杨少华 , 王 君 , 赖晓晖 , 王浩然 . LiF-BaF2-LiCl熔盐体系中Li+的电化学行为[J]. 电化学, 2016 , 22(3) : 306 -310 . DOI: 10.13208/j.electrochem.150918
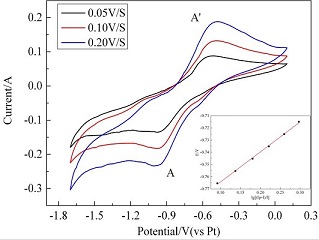
The electrochemical reduction process and rate determining step of Li ion on a tungsten electrode at 1203 K in a LiF-BaF2-LiCl molten system were studied by cyclic voltammetry, chronoamperometry and chronopotentiometry methods using a three-electrode system and electrochemical workstation AUTOLAB. The results showed that the reduction potential of Li ion was at around -1.0 V on a tungsten electrode as compared with a platinum electrode. The reduction process of Li ion on a tungsten electrode occurred in a single step with the exchange of one electron. Chronoamperograms indicated that the cathode process was controlled by the diffusion step of ions and the diffusion coefficient was calculated to be 4.5 × 10-6 cm2•s-1.
Key words: molten salt; cathode process; electrochemical; diffusion control
/
| 〈 |
|
〉 |