

金纳米粒子/聚多巴胺/碳纳米管修饰玻碳电极对核黄素的测定
收稿日期: 2015-07-27
修回日期: 2015-09-03
网络出版日期: 2015-09-06
基金资助
国家863计划资助项目(2012AA022604);国家自然科学基金(21405015,21275028);福建省自然科学基金资助项目(2014J05092);福建省教育厅项目(JA13147);大学生创新创业训练计划项目基金(201410392030)
Fabrication of Riboflavin Electrochemical Sensor Based on Au Nanoparticles/Polydopamine/Carbon Nanotubes Modified Glassy Carbon Electrode
Received date: 2015-07-27
Revised date: 2015-09-03
Online published: 2015-09-06
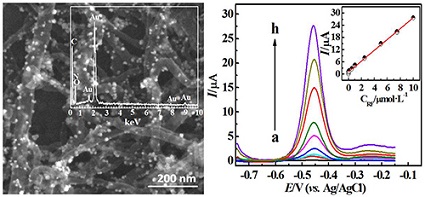
采用原位还原法制备金纳米粒子/聚多巴胺/碳纳米管(Au-PDA-MWNTs)复合材料,并将其用于建立高灵敏检测核黄素的电化学方法.采用紫外–可见光谱、扫描电镜、x-射线能谱对Au-PDA-MWNTs复合材料进行表征,采用循环伏安法和差示脉冲伏安法探讨核黄素(RF)在Au-PDA-MWNTs修饰的玻碳电极上的电化学行为,并对RF含量进行测定.该方法对核黄素的检测在5×10-9 mol·L-1~1×10-5 mol·L-1的范围内呈良好的线性关系(R=0.9906),检测限为1.7×10-9 mol·L-1.本方法操作简便、抗干扰能力强,方法可行,因此该方法成功实现了维生素药片中RF含量的测定.
彭花萍 , 余美玲 , 刘馨 , 刘盼 , 陈伟 , 刘爱林 , 林新华* . 金纳米粒子/聚多巴胺/碳纳米管修饰玻碳电极对核黄素的测定[J]. 电化学, 2016 , 22(1) : 43 -48 . DOI: 10.13208/j.electrochem.150727
A novel electrochemical platform for the high sensitivity detection of riboflavin was constructed by Au nanoparticles/polydopamine/carbon nanotubes (Au-PDA-MWCNTs) nanocomposite modified glassy carbon electrode. The Au-PDA-MWCNTs nanocomposite was synthesized by in situ reduction method. The characteristics of the as-prepared Au-PDA-MWCNTs nanocomposite modified electrodes were investigated by using UV-Vis spectroscopy, scanning electron microscopy (SEM) and electrochemical methods. Cyclic voltammetry (CV) and differential pulse voltammetry (DPV) were used to study the electrochemical behavior of riboflavin (RF) at Au-PDA-MWCNTs nanocomposite modified electrodes. The results demonstrated that the present electrochemical sensor exhibited a wide linear range from 5×10-9 mol•L-1to 1×10-5 mol•L-1 for detection of riboflavin, with a detection limit of 1.7×10-9 mol•L-1 (S/N = 3). The present method for high sensitivity determination of riboflavin by electrochemical method at Au-PDA-MWCNTs nanocomposite modified electrodes is simple, accurate, reliable and feasible with an excellent anti-interference ability against electroactive species and metal ions. Accordingly, the present method proved to be useful for the estimation of the RF content in pharmaceutical samples with satisfactory recovery.

/
| 〈 |
|
〉 |