

采用AlCl3-Emic-MgCl2室温离子液体电沉积制备铝-镁合金
收稿日期: 2014-06-03
修回日期: 2014-07-28
网络出版日期: 2015-04-23
基金资助
The authors gratefully acknowledge the Commonwealth Scholarship Commission and British Council in the UK for financial support of this work.
Electrodeposition of Al-Mg Alloys from Acidic AlCl3-Emic-MgCl2 Room Temperature Ionic Liquids
Received date: 2014-06-03
Revised date: 2014-07-28
Online published: 2015-04-23
Supported by
The authors gratefully acknowledge the Commonwealth Scholarship Commission and British Council in the UK for financial support of this work.
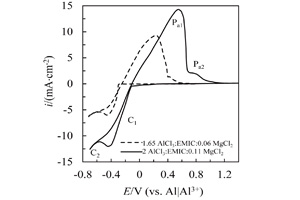
M. RostomAli , Andrew P. Abbott , Karl S. Ryder . 采用AlCl3-Emic-MgCl2室温离子液体电沉积制备铝-镁合金[J]. 电化学, 2015 , 21(2) : 172 -180 . DOI: 10.13208/j.electrochem.140603

/
| 〈 |
|
〉 |