

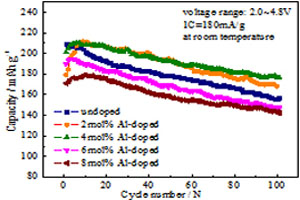
掺铝富锂材料Li1.2Mn0.543Co0.078Ni0.155Al0.030O2电极的电化学性能
收稿日期: 2013-05-16
修回日期: 2013-06-19
网络出版日期: 2014-04-17
基金资助
国家重点基础研究发展计划项目(No. 2007CB935603)资助
Effect of Al-Doping in Li-Rich Material Li1.2Mn0.543Co0.078Ni0.155Al0.030O2 for Lithium-Ion Batteries
Received date: 2013-05-16
Revised date: 2013-06-19
Online published: 2014-04-17
金晓茜 , 李益孝 , 杨勇 , 王周成 . 掺铝富锂材料Li1.2Mn0.543Co0.078Ni0.155Al0.030O2电极的电化学性能[J]. 电化学, 2014 , 20(2) : 116 -120 . DOI: 10.13208/j.electrochem.130516
/
| 〈 |
|
〉 |