

二茂铁功能化Fe3O4/碳纳米管/壳聚糖复合膜葡萄糖生物传感电极的研究
收稿日期: 2012-11-08
修回日期: 2013-01-25
网络出版日期: 2014-02-24
基金资助
国家863计划资助项目(No. 2012AA022604)、国家自然科学基金(No. 20975021,No. 21275028)、福建省高校产学研科技重点项目(No. 2010Y4003)、福建省自然科学基金资助项目(No. 2010J06011)和福建医科大学博士启动基金(No. 2011BS005)资助
Design and Development of a Novel Glucose Biosensor Based on the Ferrocene-Functionalized Fe3O4 Nanoparticles/Carbon Nanotubes/Chitosan Nanocomposite Film Modified Electrode
Received date: 2012-11-08
Revised date: 2013-01-25
Online published: 2014-02-24
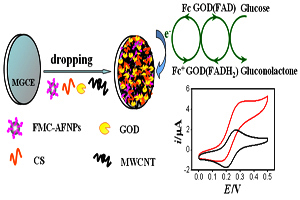
关键词: 二茂铁修饰Fe3O4; 碳纳米管; 壳聚糖; 葡萄糖氧化酶; 生物传感器
彭花萍 , 查代君 , 黄郑隽 , 陈伟 , 刘爱林 , 林新华 . 二茂铁功能化Fe3O4/碳纳米管/壳聚糖复合膜葡萄糖生物传感电极的研究[J]. 电化学, 2014 , 20(1) : 33 -38 . DOI: 10.13208/j.electrochem.121108

/
| 〈 |
|
〉 |