

多元醇法合成纳米LiCoPO4及其电化学性能
收稿日期: 2013-05-08
修回日期: 2013-05-25
网络出版日期: 2013-12-23
基金资助
安徽省自然科学基金项目(No. 1308085QB41)和上海市科研计划项目(No. 11DZ1100206)资助
Synthesis and Electrochemical Performance of Nano LiCoPO4 by Polyol Method
Received date: 2013-05-08
Revised date: 2013-05-25
Online published: 2013-12-23
王飞 , 杨军 . 多元醇法合成纳米LiCoPO4及其电化学性能[J]. 电化学, 2013 , 19(6) : 585 -589 . DOI: 10.13208/j.electrochem.130355
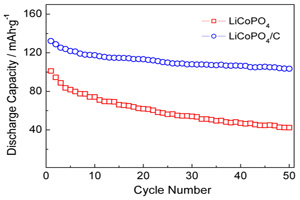
Key words: polyol method; LiCoPO4; chemical vapor deposition; carbon coating
/
| 〈 |
|
〉 |