

钒替代对碳包覆硅酸铁锂复合材料结构的影响(英文)
收稿日期: 2013-05-16
修回日期: 2013-07-08
网络出版日期: 2013-12-23
基金资助
Supported by Fujian Key Laboratory of Advanced Materials, China (No. 2006L2003).
Effect of Vanadium Substitution on Structure of Li2FeSiO4/C Composites
Received date: 2013-05-16
Revised date: 2013-07-08
Online published: 2013-12-23
Supported by
Supported by Fujian Key Laboratory of Advanced Materials, China (No. 2006L2003).
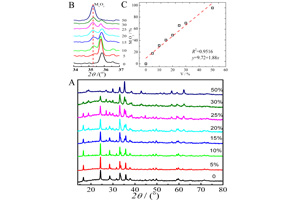
杨洪 , 张颖 , 程璇 . 钒替代对碳包覆硅酸铁锂复合材料结构的影响(英文)[J]. 电化学, 2013 , 19(6) : 565 -570 . DOI: 10.13208/j.electrochem.130356

/
| 〈 |
|
〉 |