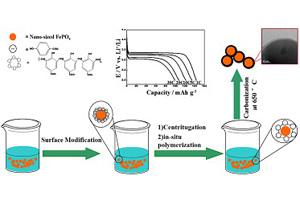
We adopted an effective route to prepare the particle size controllable core-shell structure carbon-coated LiFePO4 from different sized FePO4 precursors, varying from 80 nm, 200 nm and 1 μm by an in situ polymerization method integrated with a surface modification technology. The discharge capacities of the three sized LiFePO4/C are, respectively, 162 mAh·g-1, 142 mAh·g-1 and 92 mAh·g-1 at 0.1C rate. The nano-sized LiFePO4-a/C (80 nm) delivers a discharge capacity as large as 100 mAh·g-1 even at 30C, while the macroscopic LiFePO4-c/C (1 μm) exhibits a much poorer discharge capacity of 54 mAh·g-1 under the same current density. The carbon coated LiFePO4 (LiFePO4/C) also shows good chemical stability after the exposure to air atmosphere, in which the uniform carbon layer could prevent the LiFePO4 from reacting with H2O and O2.
[1] Padhi A K, Nanjundaswamy K S, Masquelier C, et al. Mapping of transition metal redox energies in phosphates with NASICON structure by lithium intercalation[J]. Journal of the Electrochemical Society, 1997, 144(8): 2581-2586.
[2] Lu Z G, Cheng H, Lo M F, et al. Pulsed laser deposition and electrochemical characterization of LiFePO4-Ag composite thin films[J]. Advanced Functional Materials, 2007, 17(18): 3885-3896.
[3] Huang Y H, Goodenough J B. High-rate LiFePO4 lithium rechargeable battery promoted by electrochemically active polymers[J]. Chemistry of Materials, 2008, 20(23): 7237-7241.
[4] Prosini P P, D Zane, and M Pasquali. Improved electrochemical performance of a LiFePO4-based composite cathode[J]. Electrochimica Acta, 2001, 46(23): 3517-3523.
[5] Dominko R, Bele M, Gaberscek M, et al. Impact of the carbon coating thickness on the electrochemical performance of LiFePO4/C composites[J]. Journal of the Electrochemical Society, 2005, 152(3): A607-A610.
[6] Shin H C, W I Cho, Jang H. Electrochemical properties of the carbon-coated LiFePO4 as a cathode material for lithium-ion secondary batteries[J]. Journal of Power Sources, 2006, 159(2): 1383-1388.
[7] Amin R, Lin C T, Peng J B, et al. Silicon-doped LiFePO4 single crystals: growth, conductivity behavior, and diffusivity[J]. Advanced Functional Materials, 2009, 19(11): 1697-1704.
[8] Meethong N, Kao Y H, Speakman, S A, et al. Aliovalent substitutions in olivine lithium iron phosphate and impact on structure and properties[J]. Advanced Functional Materials, 2009, 19(7): 1060-1070.
[9] Chung S Y, J T Bloking, Chiang Y M. Electronically conductive phospho-olivines as lithium storage electrodes[J]. Nature Materials, 2002, 1(2): 123-128.
[10] Liu H, Cao Q, Fu L J, et al. Doping effects of zinc on LiFePO4 cathode material for lithium ion batteries[J]. Electrochemistry Communications, 2006, 8(10): 1553-1557.
[11] Yamada A, S C Chung, Hinokuma K. Optimized LiFePO4 for lithium battery cathodes[J]. Journal of the Electrochemical Society, 2001, 148(3): A224-A229.
[12] Huang YH, K S Park, Goodenough J B. Improving lithium batteries by tethering carbon-coated LiFePO4 to polypyrrole[J]. Journal of the Electrochemical Society, 2006, 153(12): A2282-A2286.
[13] Hsu K F, S Y Tsay, Hwang B J. Synthesis and characterization of nano-sized LiFePO4 cathode materials prepared by a citric acid-based sol-gel route[J]. Journal of Materials Chemistry, 2004, 14(17): 2690-2695.
[14] Chen J, S Wang, Whittingham M S. Hydrothermal synthesis of cathode materials[J]. Journal of Power Sources, 2007, 174(2): 442-448.
[15] Yang SL, Zhou X F, Zhang J G, et al. Morphology-controlled solvothermal synthesis of LiFePO4 as a cathode material for lithium-ion batteries[J]. Journal of Materials Chemistry, 2010, 20(37): 8086-8091.
[16] Recham N, Dupont L, Courty M, et al. Ionothermal synthesis of tailor-made LiFePO4 powders for Li-ion battery applications[J]. Chemistry of Materials, 2009, 21(6): 1096-1107.
[17] Huang Y H, Ren H B; Peng Z, et al. Synthesis of LiFePO4/carbon composite from nano-FePO4 by a novel stearic acid assisted rheological phase method[J]. Electrochimica Acta, 2009, 55(1): 311-315.
[18] Lou X M, Zhang Y X. Synthesis of LiFePO4/C cathode materials with both high-rate capability and high tap density for lithium-ion batteries[J]. Journal of Materials Chemistry, 2011, 21(12): 4156-4160.
[19] Wang Y Q, Wang J L, Yang J, et al. High-rate LiFePO4 electrode material synthesized by a novel route from FePO4·4H2O[J]. Advanced Functional Materials, 2006, 16(16): 2135-2140.
[20] Oh S W, Myung S T, Oh S M, et al. Double carbon coating of LiFePO4 as high rate Electrode for rechargeable lithium batteries[J]. Advanced Materials, 2010, 22(43): 4842-4845.
[21] Wang Y G, Wang Y R, Hosono E J, et al. The design of a LiFePO4/carbon nanocomposite with a core-shell structure and its synthesis by an in situ polymerization restriction method[J]. Angewandte Chemie-International Edition, 2008, 47(39): 7461-7465.
[22] Scaccia S, Carewska M, Wisniewski P, et al. Morphological investigation of sub-micron FePO4 and LiFePO4 particles for rechargeable lithium batteries[J]. Materials Research Bulletin, 2003, 38(7): 1155-1163.
[23] Cui WJ, Li F, Liu H J, et al. Core-shell carbon-coated Cu6Sn5 prepared by in situ polymerization as a high-performance anode material for lithium-ion batteries[J]. Journal of Materials Chemistry, 2009, 19(39): 7202-7207.
[24] Porro S, Musso S, Vinante M, et al. Purification of carbon nanotubes grown by thermal CVD[J]. Physica E-Low-Dimensional Systems & Nanostructures, 2007, 37(1/2): 58-61.
[25] Martin J F, Yamada A, Kobayashi G, et al. Air exposure effect on LiFePO4[J]. Electrochemical and Solid State Letters, 2008, 11(1): A12-A16.
[26] Zaghib K, Dontigny M, Charest P, et al. Aging of LiFePO4 upon exposure to H2O[J]. Journal of Power Sources, 2008, 185(2): 698-710.
[27] Cuisinier M, Martin J F, Dupre N, et al. Moisture driven aging mechanism of LiFePO4 subjected to air exposure[J]. Electrochemistry Communications, 2010, 12(2): 238-241.