

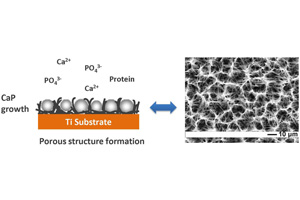
氢气气泡模板电化学诱导沉积纳-微米二级结构钙磷盐生物材料的研究
收稿日期: 2013-02-28
修回日期: 2013-04-08
网络出版日期: 2013-04-15
基金资助
国家科技支撑计划(No. 2012BAI07B09)国家自然科学基金项目(No. 51203108),江苏省自然科学基金项目(No. BK2011355)和江苏省高校自然科学研究项目(No. 11KJB430011)资助
Study on Hydrogen Bubble Template Fabrication of Porous Biomaterials Coatings by Electrochemically Induced Deposition
Received date: 2013-02-28
Revised date: 2013-04-08
Online published: 2013-04-15
王卉 , 林昌健 , 胡仁 , 张克勤 , 段红平 , 董镶 . 氢气气泡模板电化学诱导沉积纳-微米二级结构钙磷盐生物材料的研究[J]. 电化学, 2013 , 19(6) : 501 -506 . DOI: 10.13208/j.electrochem.130216
/
| 〈 |
|
〉 |