

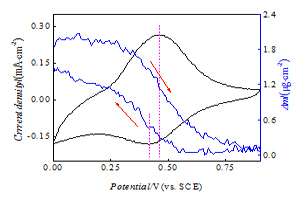
电活性铁氰化镍膜电极对稀土钇的电控离子分离
收稿日期: 2012-12-25
修回日期: 2013-03-08
网络出版日期: 2013-03-08
基金资助
国家自然科学基金项目(No. 21276173)、山西省自然科学基金项目(No. 2012011020-5,No. 2012011006-1)及山西省国际科技合作计划项目(No. 2011081028)资助
Electrochemically Switched Separation of Yttrium Ion Using Electroactive Nickel Hexacyanoferrate Thin Films in Rare Earth Metal Solution
Received date: 2012-12-25
Revised date: 2013-03-08
Online published: 2013-03-08
张浩 , 郝晓刚 , 王忠德 , 张忠林 , 马旭莉 , 李一兵 , 刘世斌 . 电活性铁氰化镍膜电极对稀土钇的电控离子分离[J]. 电化学, 2013 , 19(5) : 482 -487 . DOI: 10.61558/2993-074X.2140

/
| 〈 |
|
〉 |