

应用于水溶液介质中喹啉氧化的电芬顿工艺实验设计方法(英文)
收稿日期: 2012-12-25
修回日期: 2013-03-12
网络出版日期: 2013-03-12
Experimental Design Methodology Applied to the Oxidation of Quinolines in Aqueous Medium by Electro-Fenton Process
Received date: 2012-12-25
Revised date: 2013-03-12
Online published: 2013-03-12
TRABELSI SOUISSI Souhaila , OTURAN Nihal , BELLAKHAL Nizar , OTURAN Mehmet A . 应用于水溶液介质中喹啉氧化的电芬顿工艺实验设计方法(英文)[J]. 电化学, 2013 , 19(5) : 460 -471 . DOI: 10.61558/2993-074X.2137

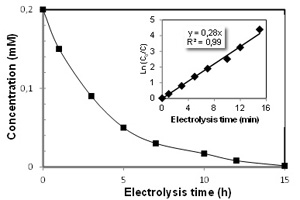
Key words: 8-HQS; electro-Fenton process; hydroxyl radicals; degradation; Doehlert matrix; mineralization; toxicity
/
| 〈 |
|
〉 |