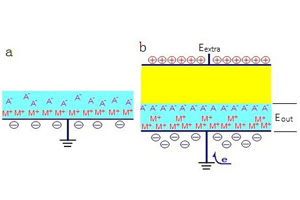
采用薄液膜实验装置,测量了外加直流电场作用下锌在薄液膜体系中的腐蚀电位、阴极极化电流以及阴极极化曲线等. 研究了外加直流电场对锌在薄液膜下腐蚀行为的影响. 结果表明,外电场的作用可以使锌电极的腐蚀电位负移,也可以使锌电极在阴极极化条件下的阴极电流增加. 分析结果表明,外电场与薄液膜体系中锌电极电化学过程中的相关因素发生了协同作用,改变了锌电极的阴极过程.
The corrosion behavior of zinc covered with thin electrolyte layers (TELs) under an application of external electric field was investigated by performing corrosion potential and cathodic polarization current measurements. The results showed that the Ecorr and ic values first increased and then decreased with the increase of electrolyte layer thickness. Pure zinc exhibited the maximum corrosion potential as the TELs increased to 400 μm under the controls of cathodic process and corrosion products. The application of external electric field resulted in either a negative shift in corrosion potential of zinc or an increase of cathodic current. The effect of external electric field on the electrode process was discussed in this paper.
[1] Chen Y(陈云), Qiang C M(强春媚), Wang G G(王国刚), et al. Corrosion and protection of transmission towers[J]. Electric Power Construction(电力建设), 2010, 31(8): 55-58.
[2] Chin D T. Corrosion by alternating current polarization of mild steel in neutral electrolytes[J]. Corrosion, 1979, 35(8): 1714-1983.
[3] Muralidharan S, Kim D K, Ha T H, et al. Influence of alternating, direct and superimposed alternating and direct current on the corrosion of mild steel in marine environments[J]. Desalination, 2007, 216 (1/3):103-115.
[4] Kim D K, Muralidharan S, Ha T H, et al. Electrochemical studies on the alternating current corrosion of mild steel under cathodic protection condition in marine environments[J]. Electrochimica Acta, 2006, 51(25): 5259-5267.
[5] Radeka R, Barisin D. Influence of frequency of alternating current on corrosion of steel in seawater[J]. Anti-Corrosion Methods and Materials, 1980, 27: 13-19.
[6] Wendt J L, Chin D T. The a.c. corrosion of stainless steel—II. The breakdown of passivity of ss304 in neutral aqueous solutions[J]. Corrosion Science, 1985, 25(10):889-900.
[7] Goidanich S, Lazzari L, Ormellese M. AC corrosion – Part 1: Effects on overpotentials of anodic and cathodic processes[J]. Corrosion Science, 2010, 52(2): 491-497.
[8] Sara G, Marco O. A theoretical study of AC-induced corrosion considering diffusion phenomena[J]. Corrosion Science, 2010, 40(2): 491-497.
[9] Gamal A E, Nishikata A, Tsuru T. AC impedence study on corrosion of 55% Al Zn alloy coated steel under thin electrolyte layers[J]. Corrosion Science, 1999, 42(7): 1509-1521.
[10] Zhang S H. Anodic processes on iron covered by thin dilute electrolyte layers anodic polarisation[J]. Corrosion Science, 1994, 36(8): 1289-1307.
[11] Nishikata A, Ichihara Y, Tsuru T. Electrochemical impedance spectroscopy of metals covered with a thin electrolyte layer[J]. Electrochim Acta, 1996, 41(7): 1057-1062.
[12] Cheng Y L, Zhang Z, Cao F H, et al. A study of the corrosion of aluminum alloy 2024-T3 under thin electrolyte layers[J]. Corrosion Science, 2004, 46(7): 1649-1667.
[13] Li M C, Jiang L L, Zhang W Q, et al. Electrochemical corrosion behavior of nanocrystalline zinc[J]. Journal of Solid State Electrochemistry, 2007, 11(5): 1319-1325.
[14]Cao C N(曹楚南). Principles of electrochemistry of corrosion (3rd)[M]. Beijing: Chemical Industry Press(化学工业出版社), 2008: 74.
[15]Tang X(唐晓), Wang J(王佳), Li Y K(李亚坤), et al. Corrosion behavior of stainless steel under NaCl electrolyte thin film[J]. Corrosion science and protection technology(腐蚀科学与防护技术), 2009, 21(3): 227-229.
[16]Huang H, Guo X, Zhang G, et al. The effects of temperature and electric field on atmospheric corrosion behaviour of PCB-Cu under absorbed thin electrolyte layer[J]. Corrosion Science, 2011, 53(5): 1700-1707.
[17]Gao D Y(高鼎拥). The calculation of electric strength in dielectric[J]. Yuxi College Journal(玉溪师专学院学报), 1986, (8): 82-86.