

Pd/Ni异结构纳米催化剂的制备及其对甲酸氧化的电催化
收稿日期: 2012-06-08
修回日期: 2012-09-05
网络出版日期: 2012-12-28
基金资助
This work was supported by the National Basic Research Program of China (973 Program) (No. 2012CB932800), the Natural Science Foundation of China (No. 21073219), Shanghai Science and Technology Committee (No. 11DZ1200400) and the Knowledge Innovation Engineering of the CAS (No. 12406, 124091231).
Electrocatalytic Oxidation of Formic Acid on Pd/Ni Heterostructured Catalyst
Received date: 2012-06-08
Revised date: 2012-09-05
Online published: 2012-12-28
Supported by
This work was supported by the National Basic Research Program of China (973 Program) (No. 2012CB932800), the Natural Science Foundation of China (No. 21073219), Shanghai Science and Technology Committee (No. 11DZ1200400) and the Knowledge Innovation Engineering of the CAS (No. 12406, 124091231).
任明军 , 邹亮亮 , 陈举 , 袁婷 , 黄庆红 , 张海峰 , 杨辉 , 封松林 . Pd/Ni异结构纳米催化剂的制备及其对甲酸氧化的电催化[J]. 电化学, 2012 , 18(6) : 515 -520 . DOI: 10.61558/2993-074X.2620

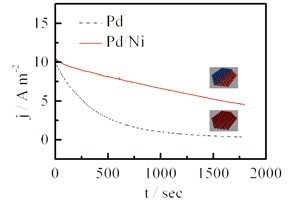
Key words: formic acid oxidation; electrocatalysis; Pd/Ni heterostructure; durability
/
| 〈 |
|
〉 |