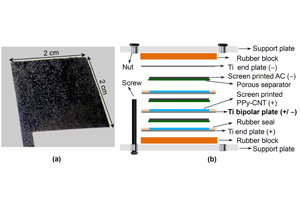
Composites of conducting polymers (polypyrrole and polyaniline) with acid treated multi-walled carbon nanotubes were formulated into printable aqueous inks, with the aid of functional additives (benzethonium chloride as a surfactant with or without polyvinyl alcohol as a binder). The inks were screen-printed as fairly uniform coatings of various mass loading densities and areas (up to 75 mg cm-2 and 100 cm2) on thin titanium plates (0.1 mm in thickness). These screen-printed plates were used to fabricate both unit cell and multi-cell stack of asymmetrical supercapacitors with screen-printed negative electrodes of activated carbon (pigment black) in aqueous electrolytes (3.0 mol L-1 KCl or 1.0 mol L-1 HCl). In particular, a three-cell stack with two bipolar Ti plates of 100 cm2 in screen-printed area was constructed, demonstrating promising technical specifications: 3.0 V in stack voltage, 1.29~1.83 F cm-2 in electrode capacitance, 2.30~3.24 Wh kg-1 in specific energy, and 1.04 kW kg-1 in maximum specific power. Cyclic voltammetry, galvanostatic charging and discharging, and electrochemical impedance spectrometry were applied to study the composites, screen-printed coatings and individual and bipolarly stacked cells, assisted by optical and electron microscopy.
[1] Arabale G, Wagh D, Kulkarni M, et al. Enhanced supercapacitance of multiwalled carbon nanotubes functionalized with ruthenium oxide[J]. Chemical Physics Letters, 2003, 376(1/2): 207-213.
[2] Simon P, Gogotsi Y. Materials for electrochemical capacitors[J]. Nature Materials, 2008, 7(11): 845-854.
[3] Eliad L, Pollak E, Levy N, et al. Assessing optimal pore-to-ion size relations in the design of porous poly(vinylidene chloride) carbons for EDL capacitors[J]. Applied Physics A: Materials Science & Processing, 2006, 82(4): 607-613.
[4] Eliad L, Salitra G, Soffer A, et al. On the mechanism of selective electroadsorption of protons in the pores of carbon molecular sieves[J]. Langmuir, 2005, 21(7): 3198-3202.
[5] Frackowiak E, Béguin F. Carbon materials for the electrochemical storage of energy in capacitors[J]. Carbon, 2001, 39(6): 937-950.
[6] Vix-Guterl C, Frackowiak E, Jurewicz K, et al. Electrochemical energy storage in ordered porous carbon materials[J]. Carbon, 2005, 43(6): p. 1293-1302.
[7] Brownson D A C, Banks C E. Fabricating graphene supercapacitors: Highlighting the impact of surfactants and moieties[J]. Chemical Communications, 2012, 48(10): 1425-1427.
[8] Peng C, Jin J, Chen G Z. A comparative study on electrochemical co-deposition and capacitance of composite films of conducting polymers and carbon nanotubes[J]. Electrochimica Acta, 2007, 53(2): 525-537.
[9] Chang J, Lee M, Tsai W, et al. Pseudocapacitive mechanism of manganese oxide in 1-ethyl-3-methylimidazolium thiocyanate ionic liquid electrolyte studied using X-ray photoelectron spectroscopy[J]. Langmuir, 2009, 25(19): 11955-11960.
[10] Conway B E. Electrochemical supercapacitors: Scientific fundamentals and technological applications[M]. New York: Kluwer Academic/Plenum. 1999.
[11] Zhang Y, Feng H, Wu X, et al. Progress of electrochemical capacitor electrode materials: A review[J]. International Journal of Hydrogen Energy, 2009, 34(11): 4889-4899.
[12] Zhou X H, Peng C, Chen G Z. 20 V stack of aqueous supercapacitors with carbon (?), titanium bipolar plates and CNT-polypyrrole composite (+)[J]. AIChE Journal, 2012, 58(3): 974-983.
[13] Frackowiak E, Khomenko V, Jurewicz K, et al. Supercapacitors based on conducting polymers/nanotubes composites[J]. Journal of Power Sources, 2006, 153(2): 413-418.
[14] Li J P, Peng T Z, Fang C. Screen-printable sol-gel ceramic carbon composite pH sensor with a receptor zeolite [J]. Analytica Chimica Acta, 2002, 455(1): 53-60.
[15] Metters J P, Kadara R O, Banks C E. New directions in screen printed electroanalytical sensors: An overview of recent developments[J]. Analyst, 2011, 136(6): 1067-1076.
[16] Ji X, Hallam P M, Houssein S M, et al. Printable thin film supercapacitors utilizing single crystal cobalt hydroxide nanosheets[J]. RSC Advances, 2012, 2(4): 1508-1515.
[17] Chen X, Xia J, Peng J, et al. Carbon-nanotube metal-matrix composites prepared by electroless plating[J]. Composites Science and Technology, 2000, 60(2): 301-306.
[18] Hu L B, Choi J W, Yang Y, et al. Highly conductive paper for energy-storage devices[J]. Proceedings of the National Academy of Sciences, 2009, 106(51):21490-21494.
[19] Hughes M, Chen G Z, Shaffer M S P, et al. Electrochemical capacitance of a nanoporous composite of carbon nanotubes and polypyrrole[J]. Chemistry of Materials, 2002, 14(4): 1610-1613.
[20] Shaffer M S P, Fan X, Windle A H. Dispersion and packing of carbon nanotubes[J]. Carbon, 1998, 36(11): 1603-1612.
[21] Wu M Q, Snook G A, Gupta V, et al. Electrochemical fabrication and capacitance of composite films of carbon nanotubes and polyaniline[J]. Journal of Materials Chemistry, 2005, 15(23): 2297-2303.
[22] Peng C, Zhang S W, Zhou X H, et al. Unequalisation of electrode capacitances for enhanced energy capacity in asymmetrical supercapacitors[J]. Energy & Environmental Science, 2010, 3(10): 1499-1502.
[23] Chae J H, Ng K C, Chen G Z, Nanostructured materials for the construction of asymmetrical supercapacitors[J]. Proceedings of the Institution of Mechanical Engineers, Part A: Journal of Power and Energy, 2010, 224(A4): 479-503.
[24] Ng K C, Zhang S W, Peng C, et al. Individual and bipolarly stacked asymmetrical aqueous supercapacitors of CNTs/SnO2 and CNTs/MnO2 nanocomposites[J]. Journal of The Electrochemical Society, 2009, 156(11): A846-A853.
[25] Hu M, Sui S, Zhu X, et al. A 10 kW class PEM fuel cell stack based on the catalyst-coated membrane (CCM) method[J]. International Journal of Hydrogen Energy, 2006, 31(8): 1010-1018.
[26] Lian K K, Li C, Jung R H, et al (Motorola, Inc.). Electrochemical cell having symmetric inorganic electrodes: United States, WO/1997/015938[P]. 1996.
[27] Staiti P, Lufrano F. Design, fabrication, and evaluation of a 1.5 F and 5 V prototype of solid-state electrochemical supercapacitor[J]. Journal of The Electrochemical Society, 2005, 152(3): A617-A621.
[28] Peng C, Hu D, Chen G Z. Theoretical specific capacitance based on charge storage mechanisms of conducting polymers: Comment on ‘Vertically oriented arrays of polyaniline nanorods and their super electrochemical properties’[J]. Chemical Communications, 2011, 47(14): 4105-4107.



