

金(111)电极表面浮动磷脂双层膜的原位偏振红外反射光谱研究
收稿日期: 2012-08-25
修回日期: 2012-10-23
网络出版日期: 2012-10-28
基金资助
This work was supported by Discovery grant from the Natural Sciences and Engineering Research Council of Canada. J.L. acknowledges support from the Canada Research Chairs (CRC) program.
In situ PM-IRRAS Studies of a Floating Bilayer Lipid Membrane at Au(111) Electrode Surface
Received date: 2012-08-25
Revised date: 2012-10-23
Online published: 2012-10-28
Supported by
This work was supported by Discovery grant from the Natural Sciences and Engineering Research Council of Canada. J.L. acknowledges support from the Canada Research Chairs (CRC) program.
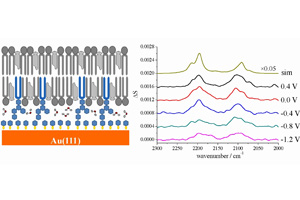
关键词: 原位偏振红外反射光谱; 金(111)电极; 浮动磷脂双层膜
Zhangfei Su , Annia Kycia , J. Jay Leitch , Jacek Lipkowski . 金(111)电极表面浮动磷脂双层膜的原位偏振红外反射光谱研究[J]. 电化学, 2012 , 18(5) : 387 -404 . DOI: 10.61558/2993-074X.2610

/
| 〈 |
|
〉 |