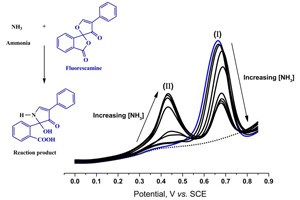
荧光胺是一种非荧光剂,其易与伯胺反应形成荧光产物,被普遍用于伯胺的荧光光谱定量分析. 本文利用荧光胺与伯胺反应发展了一种新型灵敏的伏安法用于检测水溶液中的伯胺. 首先,在有、无伯胺的0.1 mol L-1 PBS (pH 9.0)缓冲液中,研究了玻碳电极表面荧光胺的循环伏安电化学行为. 荧光胺的不可逆氧化峰出现在0.70 V (vs. SCE),当加入伯胺时,在0.46 V (vs. SCE)出现另一不可逆的氧化峰,为荧光胺与伯胺反应的产物. 继续加入氨水,荧光胺的氧化峰变弱,反应产物的氧化峰则由于荧光胺按反应化学计量比随氨消耗增多而随之增大. 上述两个阳极峰分别对应于荧光胺及其反应产物,采用方波伏安和荧光光谱技术可实现水溶液中伯胺的定量检测. 在0 ~ 60 μmol L-1氨浓度范围内,该反应产物方波伏安检测成线性响应. S/N = 3或3σ时检测下限分别为0.71 μmol L-1和3.17 μmol L-1,与荧光法检测的结果相近.
Janjira Panchompoo
,
Richard G. Compton
. 荧光胺电化学检测水溶液中的氨:荧光检测与伏安分析比较[J]. 电化学, 2012
, 18(5)
: 437
-449
.
DOI: 10.61558/2993-074X.2614
Fluorescamine is a non-fluorescent reagent widely used for the quantitative determination of primary amines by fluorescence spectroscopy as it reacts readily with primary amines to form a fluorescent product. In this work, a new sensitive voltammetric method for the detection of ammonia in aqueous solution by the reaction with fluorescamine has been developed. First, the electrochemical behaviour of fluorescamine in the absence and presence of ammonia was investigated in 0.1 mol L-1 borate buffer solution (pH 9.0) by cyclic voltammetry using a glassy carbon (GC) electrode. As for fluorescamine itself, a well-defined irreversible oxidation peak could be observed at ca. 0.70 V vs. SCE. When ammonia was added to the fluorescamine solution, another irreversible oxditaion peak corresponding to the oxidation of the reaction product formed between fluorescamine and ammonia could be observed at ca. 0.46 V vs. SCE. Upon the addition of ammonia, the oxidation peak of fluorescamine became smaller while the oxidation peak of the reaction product formed increased in height, due to the stoichiometric chemical consumption of fluorescamine by ammonia and the formation of the product during the reaction, respectively. These two anodic peaks corresponding to the oxidation of fluorescamine and its fluorescent product formed were then used for the quantitative detection of ammonia, explored by square wave voltammetry and by fluorescence spectroscopy. The square wave voltammetric response of the reaction product formed showed a linear response over ammonia concentration range of 0 to 60 μmol L-1. The limits of detection (LOD) was found to be 0.71 μmol L-1 and 3.17 μmol L-1 determined based upon Signal/Noise (S/N) = 3 and 3σ, respectively. These limits of detection are similar to those obtained with the fluorometric method.