

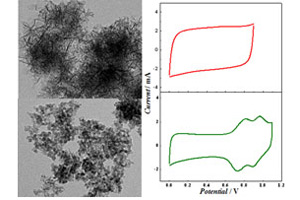
纳米MnO2的制备及其电化学性能研究
收稿日期: 2011-11-19
修回日期: 2012-02-24
网络出版日期: 2012-02-29
基金资助
国家自然科学基金重点项目(No. 20633040)和纳米重大科学研究计划(No. 2011CB935904)资助
Preparation and Electrochemical Performance of Nanostructured MnO2 Materials
Received date: 2011-11-19
Revised date: 2012-02-24
Online published: 2012-02-29
吴雯 , 周丹丹 , 侯孟炎 , 夏永姚 . 纳米MnO2的制备及其电化学性能研究[J]. 电化学, 2012 , 18(4) : 295 -300 . DOI: 10.61558/2993-074X.2919
/
| 〈 |
|
〉 |