

YF3包覆Li(Li0.22Ni0.17Mn0.61)O2正极材料的性能
收稿日期: 2011-11-24
修回日期: 2012-02-16
网络出版日期: 2012-02-21
基金资助
国家973计划(No. 2009CB220100)资助
Electrochemical Performance of YF3-Coated Li(Li0.22Ni0.17Mn0.61)O2 Cathode Material for Li-Ion Batteries
Received date: 2011-11-24
Revised date: 2012-02-16
Online published: 2012-02-21
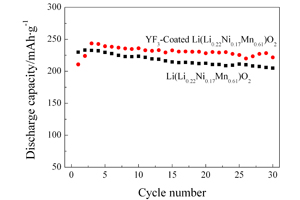
冯欣 , 李国然 , 叶世海 , 高学平 . YF3包覆Li(Li0.22Ni0.17Mn0.61)O2正极材料的性能[J]. 电化学, 2012 , 18(4) : 322 -327 . DOI: 10.61558/2993-074X.2925

/
| 〈 |
|
〉 |