

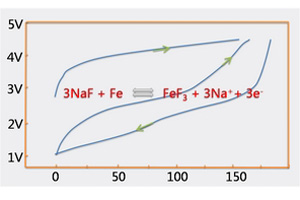
NaF-M(M=Fe, Cu)钠离子电池转换正极材料的研究
收稿日期: 2011-11-24
修回日期: 2012-02-11
网络出版日期: 2012-02-16
基金资助
国家自然科学基金项目(No. 2009CB2201003)资助
NaF-M (M = Fe, Cu) Nanocomposites as Conversion Cathode Materials for Sodium Ion Batteries
Received date: 2011-11-24
Revised date: 2012-02-11
Online published: 2012-02-16
李婷 , 陈重学 , 曹余良 , 杨汉西 . NaF-M(M=Fe, Cu)钠离子电池转换正极材料的研究[J]. 电化学, 2012 , 18(4) : 291 -294 . DOI: 10.61558/2993-074X.2918
/
| 〈 |
|
〉 |