

功率型锂离子电池负极材料TiO2/石墨烯的制备及其电化学性能研究
收稿日期: 2012-04-30
修回日期: 2012-05-10
网络出版日期: 2012-05-15
基金资助
国家自然科学基金项目(No. 200933005, No. 02903077),国家972项目(No. 2009CB220102)资助和厦门市纯电动汽车重大科技专项(I期)(No. 3502Z20121002)
Preparation and Electrochemical Performance of TiO2/GNs Nanocomposite as Anode Materials for Lithium-Ion Batteries
Received date: 2012-04-30
Revised date: 2012-05-10
Online published: 2012-05-15
秦琳琳 , 张焕 , 刘晓静 , 许剑辉 , 施一宁 , 郑明森 , 董全峰 . 功率型锂离子电池负极材料TiO2/石墨烯的制备及其电化学性能研究[J]. 电化学, 2012 , 18(3) : 201 -204 . DOI: 10.61558/2993-074X.2904
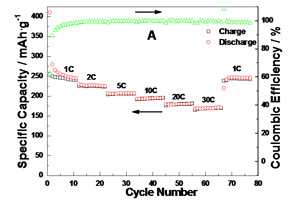
Key words: lithium-ion battery; anode materials; TiO2; graphene
/
| 〈 |
|
〉 |