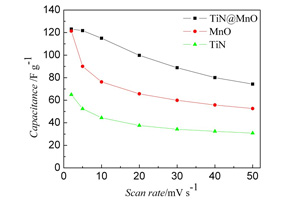
本文以钛酸四丁酯和乙酰丙酮锰为起始原料,聚乙烯吡咯烷酮(PVP)为分散剂分别配制高分子溶液. 采用同轴静电纺丝法制备了TiN@MnO前驱体,并经氨气处理得到了具有芯-壳结构的TiN@MnO同轴纤维. 采用X射线衍射(XRD)、场发射扫描电子显微镜(FESEM)、透射电子显微镜(TEM)、X射线能量色散谱(EDX)和物理吸附仪分析、观察和表征TiN@MnO同轴结构纤维,其比表面积达16 m2?g-1. 循环伏安曲线测试(CV)表明,在20 mV?s-1倍率下,TiN@MnO同轴纤维电极比电容保持率为2 mV?s-1倍率下的81%,充分说明TiN和MnO两种组分的协同效应提高了电极的倍率性能.
商超群
,
杨海燕
,
周新红
,
满忠雷
,
韩鹏献
,
姚建华
,
段玉龙
,
崔光磊
. 同轴静电纺丝法制备TiN@MnO纤维及其电化学性能研究[J]. 电化学, 2012
, 18(3)
: 257
-263
.
DOI: 10.61558/2993-074X.2912
In this study, the titanium nitride (TiN) @manganese oxide (MnO) core-shell structured fibers were prepared by the coaxial electrospinning using tetrabutyl titanate and manganese acetylacetonate as raw materials, and polyvinylpyrrolidone (PVP) as the template. And then the fibers were annealed in ammonia to finally obtain the coaxial TiN@MnO fibers. XRD, FESEM, TEM, EDX and physical adsorption instrument were used to characterize the phase structure, morphology, composition and specific surface areas and pore sizes of the samples. It was demonstrated that the as-synthesized TiN@MnO fibers possessed coaxial structure with a surface area of 16 m2?g-1. As indicated from the cyclic voltammetry test, the capacitances of these fibers displayed 100 F?g-1(TiN 38F?g-1, MnO 66 F?g-1)at a scan rate of 20 mV?s-1 and 82 F?g-1 at a higher rate of 50 mV?s-1, which were resulted by efficiently combining the large capacitance of MnO with good electronic conductivity of TiN.
[1] Zhao Y, Jiang L. Hollow micro/nanomaterials with multilevel interior structures[J]. Advanced Materials, 2009, 21(36): 3621-3638.
[2] Ji L W, Zhang X W. Manganese oxide nanoparticle-loaded porous carbon nano?bers as anode materials for high-performance lithium-ion batteries[J]. Electrochemistry Communications, 2009, 11(4): 795-798.
[3] Zhao Y, Cao X Y, Jiang L. Bio-mimic multichannel microtubes by a facile method[J]. Journal of the American Chemical Society, 2007, 129(4): 764-765.
[4] Dong S M, Chen X, Cui G L, et al. One dimensional MnO2/titanium nitride nanotube coaxial arrays for high performance electrochemical capacitive energy storage[J]. Energy & Environmental Science, 2011, 4(9): 3502-3508.
[5] Yan J A, Khoo E, Lee P S, et al. Facile coating of manganese oxide on tin oxide nanowires with high-performance capacitive behavior[J]. ACS Nano, 2010, 4(7): 4247-4255.
[6] Liu R, Lee S B. MnO2/Poly(3,4-ethylenedioxythiophene) coaxial nanowires by one-step coelectrodeposition for electrochemical energy storage[J]. Journal of the American Chemical Society, 2008, 130(10): 2942-2943.
[7] Bi R R, Guo Y G, Wan L J, et al. Synthesis of flake-like MnO2/CNT composite nanotubes and their applications in electrochemical capacitors[J]. Journal of Nanoscience and Nanotechnology, 2011, 11(3): 1996-2002.
[8] Zhou X H, Shang C Q, Cui G L, et al. Mesoporous coaxial titanium nitride-vanadium nitride fibers of core-shell structures for high-performance supercapacitors[J]. ACS applied materials & interfaces, 2011, 3(8): 3058-3063.
[9] Binotto G, Larcher D, Tarascon J M, et al. Synthesis, characterization, and Li-electrochemical performance of highly porous Co3O4 powders[J]. Chemistry of Materials, 2007, 19(12): 3032-3040.
[10] Lou X W, Deng D, Archer L A, et al. Self-supported formation of needlelike Co3O4 nanotubes and their application as lithium-ion battery electrodes[J]. Advanced Materials, 2008, 20(2): 258-262.
[11] Zhou W, Cheng C, Fan H J, et al. Epitaxial growth of branched α-Fe2O3/SnO2 nano-heterostructures with improved lithium-ion battery performance[J]. Advanced Functional Materials, 2011, 21 (13): 2439-2445.
[12] Liu Y M, Zhao X Y, Xia D G, et al. Facile synthesis of MnO/C anode materials for lithium-ion batteries[J]. Electrochimica Acta, 2011, 56(18): 6448-6452.
[13] Fang X, Lu X, Chen L, et al. Electrode reactions of manganese oxides for secondary lithium batteries[J]. Electrochemistry Communications, 2010, 12(11): 1520-1523.
[14] Pereira N, Dupont L, Amatucci G G, et al. Electrochemistry of Cu3N with lithium[J]. Journal of the Electrochemical Society, 2003, 150(9): A1273-1280.
[15] Choi D, Blomgren G E, Kumta P N. Fast and reversible surface redox reaction in nanocrystalline vanadium nitride supercapacitors[J]. Advanced Materials, 2006, 18(9): 1178-1182.
[16] Zhou X, Chen H, Nan J, et al. Study on the electrochemical behavior of vanadium nitride as a promising supercapacitor material[J]. Journal of Physics and Chemistry of Solids, 2009, 70(2): 495-500.
[17] Liu T C, Pell W G, Conway B E, et al. Behavior of molybdenum nitrides as materials for electrochemical capacitors-Comparison with ruthenium oxide[J]. Journal of the Electrochemical Society, 1998, 145(6): 1882-1888.
[18] Dong S M, Chen X, Cui G L, et al. Facile preparation of mesoporous titanium nitride microspheres for electrochemical energy storage[J]. ACS Applied Materials & Interfaces, 2011, 3(1): 93-98.
[19] Cui G L, Gu L, Maier J, et al. A carbon/titanium vanadium nitride composite for lithium storage[J]. ChemPhysChem, 2010, 11(15): 3219-3223.
[20] Chen W, Fan Z L, Wang C L, et al. Enhanced capacitance of manganese oxide via confinement inside carbon nanotubes[J]. Chemical Communications, 2010, 46(22): 3905-3907.
[21] Wang L, Wang H B, Cui G L, et al. A facile method of preparing mixed conducting LiFePO4/Graphene composites for lithium ion batteries[J]. Solid State Ionics, 2010, 181(37/38): 1685-1689.
[22] Pang S C, Anderson M A, Chapman T W. Novel electrode materials for thin-film ultracapacitors: Comparison of electrochemical properties of sol-gel-derived and electrodeposited manganese dioxide[J]. Journal of the Electrochemical Society, 2000, 147(2): 444-450.
[23] Era A, Takehara Z, Yoshizawa S. Discharge mechanism of the manganses oxide electrode[J]. Electrochimica Acta, 1967, 12(9): 1199-1212.