

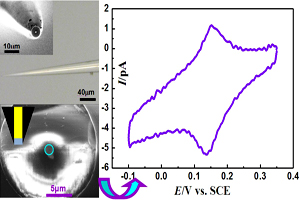
普鲁士蓝纳米修饰电极的制备及表征
收稿日期: 2011-12-02
修回日期: 2012-03-23
网络出版日期: 2012-03-25
基金资助
国家自然科学基金项目(No. 20973142),中美NSFC-NSF化学领域合作项目(No. 21061120456)和国家自然科学基金委界面电化学创新群体(No. 21021002)资助
Preparation and Characterization of Prussian Blue Modified Nanoelectrode
Received date: 2011-12-02
Revised date: 2012-03-23
Online published: 2012-03-25
王玮 , 苏宝法 , 詹东平 . 普鲁士蓝纳米修饰电极的制备及表征[J]. 电化学, 2012 , 18(3) : 252 -256 . DOI: 10.61558/2993-074X.2911
/
| 〈 |
|
〉 |