

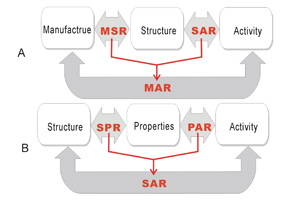
“性质-活性关系”对催化剂研究的方法论意义
收稿日期: 2011-11-21
修回日期: 2012-03-14
网络出版日期: 2012-03-16
基金资助
国家自然科学基金项目(No. 20933004,No. 21125312)和国家973计划项目(No. 2012CB932800,No. 2012CB215503)资助
Methodological Significance of “Property-Activity Relationship” for Catalyst Studies
Received date: 2011-11-21
Revised date: 2012-03-14
Online published: 2012-03-16
陆君涛 , 肖丽 , 王得丽 , 孙玉宝 , 索艳格 , 庄林 . “性质-活性关系”对催化剂研究的方法论意义[J]. 电化学, 2012 , 18(3) : 215 -222 . DOI: 10.61558/2993-074X.2906

/
| 〈 |
|
〉 |