

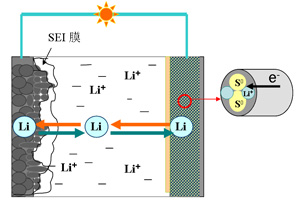
锂-硫二次电池界面反应的特殊性与对策分析
收稿日期: 2011-11-08
修回日期: 2012-02-20
网络出版日期: 2012-02-25
基金资助
国家973计划项目(No. 2009CB220103)资助
Simple Analysis and Possible Solutions of the Unusual Interfacial Reactions in Li-S batteries
Received date: 2011-11-08
Revised date: 2012-02-20
Online published: 2012-02-25
艾新平 , 曹余良 , 杨汉西 . 锂-硫二次电池界面反应的特殊性与对策分析[J]. 电化学, 2012 , 18(3) : 224 -228 . DOI: 10.61558/2993-074X.2907
/
| 〈 |
|
〉 |