

阵列纳米通道中氨基官能团质子化研究
收稿日期: 2011-12-23
修回日期: 2012-02-22
网络出版日期: 2012-03-02
基金资助
973项目(No. 2012CB933804),国家自然科学基金项目(No. 21035002),高等学校博士点基金(No. 200802840012)和江苏省自然科学基金攀登计划(No. BK2010009)资助
Protonization of Amino Functional Groups Confined in Nanochannels
Received date: 2011-12-23
Revised date: 2012-02-22
Online published: 2012-03-02
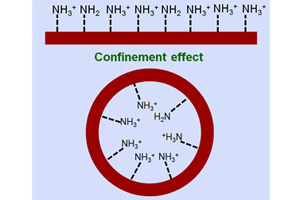
高红丽 , 周凯琳 , 王琛 , 李素娟 , 章慧 , 夏兴华 . 阵列纳米通道中氨基官能团质子化研究[J]. 电化学, 2012 , 18(3) : 229 -234 . DOI: 10.61558/2993-074X.2908

/
| 〈 |
|
〉 |