

电化学去合金化Pt(Pd)-Cu对氧的电催化还原活性的研究
收稿日期: 2011-11-24
修回日期: 2012-01-09
网络出版日期: 2012-01-19
基金资助
苏州大学青年教师科学基金(No. SDY2011A04)和美国能源部基金(No. DE-AC02-76SF00515; No. LAB04-20)资助
Catalytic Activities of Electrochemically Dealloyed Pt(Pd)-Cu Catalysts for Oxygen Reduction Reaction
Received date: 2011-11-24
Revised date: 2012-01-09
Online published: 2012-01-19
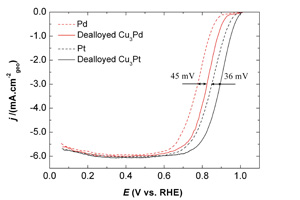
关键词: 氧气电化学还原反应; 电化学去合金; Pt(Pd)-Cu合金; 电催化剂
杨瑞枝 , Peter Strasser , Michael Toney . 电化学去合金化Pt(Pd)-Cu对氧的电催化还原活性的研究[J]. 电化学, 2012 , 18(2) : 141 -146 . DOI: 10.61558/2993-074X.2895

/
| 〈 |
|
〉 |