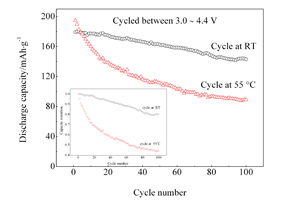
The Ni-rich cathode materials, LiNi0.5Co0.2Mn0.3O2, have been synthesized by Co-precipitation and high-temperature solid-phase sintering method. Constant current charge-discharge tests showed high discharge capacity of 179.2 mAh.g-1 in the 3.0 ? 4.4 V at 0.2C. However, at 55 °C the LiNi0.5Co0.2Mn0.3O2 experienced the dramatic capacity fading after 100 charge-discharge cycles. Electrochemical Impedance Spectroscopy, X-Ray Photoelectron Spectroscopy, Atomic Emission Spectroscopy have been employed to study the capacity fading mechanism of LiNi0.5Co0.2Mn0.3O2 cycled at high temperature in range of high-voltage charge and discharge conditions. It was found that at high temperature under conditions of high-voltage range, the side reactions between the electrolyte and electrode would be accelerated, leading to dissolution of transition metal atoms and resulting in the local structure damage of cathode material. Meanwhile, the byproducts could be deposited on the electrode surface as a high impedance LiF/metal fluoride layer, the charge-transfer resistance and Li+ diffusion resistance were increased, resulting in a sharp capacity degradation.
[1] Dahn J R, Von Sacken U, Michal C A. Structure and electrochemistry of Li1±yNiO2 and a new Li2NiO2 phase with the Ni (OH)2 structure [J]. Solid State Ionics, 1990, 44(1/2): 87-97.
[2] Liu Z, Yu A, Lee J Y. Synthesis and characterization of LiNi1-x-yCoxMnyO2 as the cathode materials of secondary lithium batteries [J]. Journal of Power Sources, 1999, 81-82: 416-419.
[3] Andersson A M, Edstro?m K. Chemical composition and morphology of the elevated temperature SEI on graphite [J]. Journal of the Electrochemical Society, 2001, 148(10): A1100-A1109.
[4] Liu H. A comparative study of LiNi0.8Co0.2O2 cathode materials modified by lattice-doping and surface-coating [J]. Solid State Ionics, 2004, 166(3/4): 317-325.
[5] Chernova N A, Ma M, Xiao J, et al. Layered LixNiyMnyCo1-2yO2 cathodes for lithium ion batteries:? understanding local structure via magnetic properties [J]. Chemistry of Materials, 2007, 19(19): 4682-4693.
[6] Ni J, Zhou H, Chen J, et al. Improved electrochemical performance of layered LiNi0.4Co0.2Mn0.4O2 via Li2ZrO3 coating [J]. Electrochimica Acta, 2008, 53(7): 3075-3083.
[7] Huang Y, Chen J, Cheng F, et al. A modified Al2O3 coating process to enhance the electrochemical performance of Li(Ni1/3Co1/3Mn1/3)O2 and its comparison with traditional Al2O3 coating process [J]. Journal of Power Sources, 2010, 195(24): 8267-8274.
[8] Abraham D P, Twesten R D, Balasubramanian M, et al. Surface changes on LiNi0.8Co0.2O2 particles during testing of high-power lithium-ion cells [J]. Electrochemistry Communications, 2002, 4(8): 620-625.
[9] Kobayashi H, Shikano M, Koike S, et al. Investigation of positive electrodes after cycle testing of high-power Li-ion battery cells [J]. Journal of Power Sources, 2007, 174(2): 380-386.
[10] Sun Y, Myung S, Yoon C S, et al. Improvement of high voltage cycling performances of LiNi1/3Co 1/3Mn1/3O2 at 55 °C by a (NH4)3AlF6 coating [J]. Electrochemical and Solid State Letters, 2009, 12(8): A163-A166.
[11] Lee Y S, Kim S B, Lee K J, et al. Preparation and cycle performance at high temperature for Li[Ni0.5Co0.2Mn0.3]O2 coated with LiFePO4 [J]. Journal of Solid-State Electrochemistry, 2010, 14(6): 919-922.
[12] Lee Y S, Kim W S, Kim S B, et al. Remarkable improvement in cell safety for Li[Ni0.5Co0.2Mn0.3]O2 coated with LiFePO4 [J]. Journal of Alloys and Compounds, 2010, 492(1/2): L87-L90.
[13] Liu D, Lu Y, Goodenough J B. Rate properties and elevated-temperature performances of LiNi0.5?xCr 2xMn1.5?xO4 (0≤2x≤0.8) as 5 V cathode materials for lithium-ion batteries [J]. Journal of the Electrochemical Society, 2010, 157(11): A1269-A1273.
[14] Park M, Lee J, Choi W, et al. On the surface modifications of high-voltage oxide cathodes for lithium-ion batteries: new insight and significant safety improvement [J]. Journal of Materials Chemistry, 2010, 20(34): 7208-7213.
[15] Lee D, Scrosati B, Sun Y. Ni3(PO4)2-coated Li[Ni0.8Co0.15Al0.05]O2 lithium battery electrode with improved cycling performance at 55 °C [J]. Journal of Power Sources, 2011, 196(18): 7742-7746.
[16] Sun Y, Lee B, Noh H, et al. A novel concentration-gradient Li[Ni0.83Co0.07Mn0.10]O2 cathode material for high-energy lithium-ion batteries [J]. Journal of Materials Chemistry, 2011, 21(27): 10108-10112.
[17] Lee B R, Noh H J, Myung S T, et al. High-Voltage Performance of Li[Ni0.55Co0.15Mn0.30]O2 positive electrode material for rechargeable Li-ion batteries [J]. Journal of the Electrochemical Society, 2011, 158(2): A180-A186.
[18] Edstrom K, Gustafsson T, Thomas J. The cathode–electrolyte interface in the Li-ion battery [J]. Electrochimica Acta, 2004, 50(2/3): 397-403.
[19] Chen Z Y (陈召勇), Liu X Q (刘兴泉), Gao L Z (高利珍), et al. Electrochemical capacity fading in high temperature of spinel LiMn2O4 and its improvement [J]. Chinese Journal of Inorganic Chemistry (无机化学学报), 2001, 17(3): 325-330.
[20] Nobili F, Croce F, Scrosati B, et al. Electronic and electrochemical properties of LixNi1-yCoyO2 cathodes studied by impedance spectroscopy [J]. Chemistry of Materials, 2001, 13(5): 1642-1646.
[21] Zhang S S, Xu K, Jow T R. Electrochemical impedance study on the low temperature of Li-ion batteries [J]. Electrochimica Acta, 2004, 49(7): 1057-1061.
[22] Verma P, Maire P, Novák P. A review of the features and analyses of the solid electrolyte interphase in Li-ion batteries [J]. Electrochimica Acta, 2010, 55(22): 6332-6341.
[23] Murakami M, Yamashige H, Arai H, et al. Direct evidence of LiF Formation at electrode/electrolyte interface by 7Li and 19F double-resonance solid-state NMR spectroscopy [J]. Electrochemical and Solid-State Letters, 2011, 14(9): A134-A137.
[24] Dahe?ron L, Martinez H, Dedryve?re R, et al. Surface properties of LiCoO2 investigated by XPS analyses and theoretical calculations [J]. The Journal of Physical Chemistry C, 2009, 113(14): 5843-5852.
[25] Myung S, Amine K, Sun Y. Surface modification of cathode materials from nano- to microscale for rechargeable lithium-ion batteries [J]. Journal of Materials Chemistry, 2010, 20(34): 7074-7095.



