

Sn/Cu电极电化学还原CO2的研究
收稿日期: 2011-10-31
修回日期: 2011-12-09
网络出版日期: 2011-12-16
基金资助
国家自然科学基金(50873050)和国家“973”项目(2011CB935902, 2012CB215501)资助
Electrochemical Reduction of CO2 on Sn/Cu Electrodes
Received date: 2011-10-31
Revised date: 2011-12-09
Online published: 2011-12-16
赵晨辰 , 郭建伟 , 王莉 , 何向明 , 王诚 , 刘志祥 . Sn/Cu电极电化学还原CO2的研究[J]. 电化学, 2012 , 18(2) : 169 -173 . DOI: 10.61558/2993-074X.2899
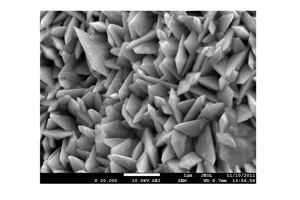
Key words: Sn/Cu; electrode; electrochemical reduction of CO2
/
| 〈 |
|
〉 |