

(PhMgCl)2-AlCl3在混合醚溶剂中的电化学性能研究
收稿日期: 2011-11-03
修回日期: 2011-11-30
网络出版日期: 2011-12-08
基金资助
国家重点基础研究发展计划973课题(2007CB209700)资助
Electrochemical Characterization of (PhMgCl)2-AlCl3/Mixed Ether Electrolytes
Received date: 2011-11-03
Revised date: 2011-11-30
Online published: 2011-12-08
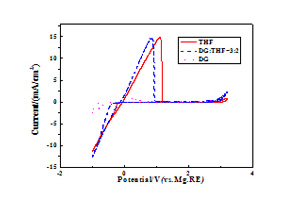
王菲菲 , 郭永胜 , 杨军 , 努丽燕娜 , 王久林 . (PhMgCl)2-AlCl3在混合醚溶剂中的电化学性能研究[J]. 电化学, 2012 , 18(1) : 56 -61 . DOI: 10.61558/2993-074X.2880

/
| 〈 |
|
〉 |