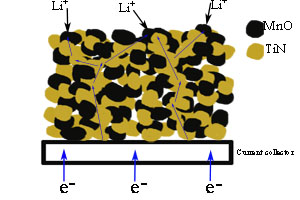
以乙酸锰和钛酸四丁酯为原料,柠檬酸为络合剂,采用溶胶-凝胶法制备钛酸锰(MnTiO3)粉体,而后将其粉体高温氨气氮化,可得到MnO/TiN复合材料. 使用X射线衍射(XRD)、X射线能量色散谱(EDS)和场发射扫描电子显微镜(FESEM)表征材料的物相结构与组分、观察其形貌. 采用循环伏安、恒流充放电和电化学阻抗方法测试电极电化学性能. 结果表明,MnO/TiN电极在100 mA?g-1和1 A?g-1倍率放电下,比容量分别为394 mAh?g-1和146 mAh?g-1,均高于单纯MnO电极比容量和倍率性能,这归因于复合材料中的TiN提供了导电网络,并有效地抑制了电极在充放电过程中的体积膨胀效应.
许高洁
,
杨海燕
,
韩鹏献
,
张立学
,
张克军
,
张传健
,
范玉华
,
毕彩丰
,
崔光磊
. 锂离子电池MnO/TiN负极研究[J]. 电化学, 2012
, 18(1)
: 37
-42
.
DOI: 10.61558/2993-074X.2877
Manganese titanate (MnTiO3) powder was prepared via a sol-gel method using manganese acetate and tetrabutyl titanate as raw materials, and citric acid as a chelating agent. Through high temperature nitridation of MnTiO3 powder in ammonia, the MnO/TiN composite was obtained. The phase structure, composition and morphology of the composites were characterized by XRD, SEM and EDS. The electrochemical properties of the composite electrodes were studied by performing cyclic voltammetry, galvanostatic charge and discharge, and electrochemical impedance spectroscopy tests. The MnO/TiN electrode delivered specific capacities of 394 mAh?g-1 and 146 mAh?g-1 at the current density of 100 mA?g-1 and 1 A?g-1, respectively, and exhibited higher specific capacity and superior rate capability than MnO electrode, which can be ascribed to the presence of TiN in the composite offering an electron conducting network and suppressing the volume expansion of MnO efficiently during the charge and discharge processes.