

中温固体氧化物燃料电池LaNi0.6Fe0.4O3-δ阴极材料的制备及性能表征
收稿日期: 2011-08-19
修回日期: 2011-09-09
网络出版日期: 2011-10-20
基金资助
教育部高等学校博士学科点专项科研基金(20100073120055)资助
Preparation and Characterization of the LaNi0.6Fe0.4O3-δ Cathode for Intermediate Temperature Solid Oxide Fuel Cell
Received date: 2011-08-19
Revised date: 2011-09-09
Online published: 2011-10-20
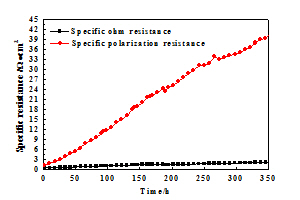
刘珩 , 黄波 , 朱新坚 . 中温固体氧化物燃料电池LaNi0.6Fe0.4O3-δ阴极材料的制备及性能表征[J]. 电化学, 2011 , 17(4) : 421 -426 . DOI: 10.61558/2993-074X.2865

Key words: solid oxide fuel cell; cathode; Fe-Cr alloy; Cr deposition; impedance spectroscopy
/
| 〈 |
|
〉 |