

掺杂LiMxV3-xO8正极材料的电化学性能
收稿日期: 2011-02-11
修回日期: 2011-05-26
网络出版日期: 2011-07-30
基金资助
国家自然基金(20663006)
The Electrochemical Properties of Cathode Materials LiMxV3-xO8
Received date: 2011-02-11
Revised date: 2011-05-26
Online published: 2011-07-30
杨辉 , 赵刚刚 , 李娟 . 掺杂LiMxV3-xO8正极材料的电化学性能[J]. 电化学, 2011 , 17(4) : 399 -404 . DOI: 10.61558/2993-074X.2861
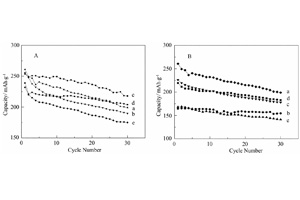
Key words: lithium ion battery; nanomaterial; LiV3O8; electrochemical properties; doping; sol-gel
/
| 〈 |
|
〉 |