

阵列参比电极法研究316不锈钢焊缝腐蚀行为
Corrosion Behaviors of 316 Stainless Steel Weldment Studied by Array Reference Electrodes
Received date: 2011-04-27
Revised date: 2011-05-09
Online published: 2011-06-09
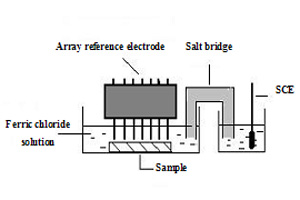
杨旺火 , 胡融刚 , 叶陈清 , 杭纬 , 李宁 , 林昌健 . 阵列参比电极法研究316不锈钢焊缝腐蚀行为[J]. 电化学, 2011 , 17(4) : 373 -379 . DOI: 10.61558/2993-074X.2857

/
| 〈 |
|
〉 |