

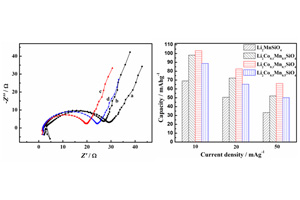
锂离子电池正极材料 Li2CoxMn1-xSiO4的合成及电化学性能研究
收稿日期: 2011-03-07
修回日期: 2011-03-15
网络出版日期: 2011-06-09
基金资助
国家自然科学基金(基金号:20873115 )、国家重点基础研究发展规划973课题(2007CB209702)
Synthesis and Electrochemical Behaviors of Li2CoxMn1-xSiO4 as a Cathode Material for Lithium-Ion Batteries
Received date: 2011-03-07
Revised date: 2011-03-15
Online published: 2011-06-09
王琳 , 吕东平 , 杨勇 . 锂离子电池正极材料 Li2CoxMn1-xSiO4的合成及电化学性能研究[J]. 电化学, 2011 , 17(3) : 318 -322 . DOI: 10.61558/2993-074X.2847
/
| 〈 |
|
〉 |