

石墨烯对Ni(OH)2超级电容器材料的电化学行为的影响
收稿日期: 2011-04-18
修回日期: 2011-05-24
网络出版日期: 2011-06-09
基金资助
国家自然科学基金(20821063和20873063);973项目基金(2010CB732400)
Effects of Graphene on Electrochemical Behaviors of Ni(OH)2 as Supercapacitor Material
Received date: 2011-04-18
Revised date: 2011-05-24
Online published: 2011-06-09
赵真真 , 倪文彬 , 高能越 , 王洪波 , 赵健伟 . 石墨烯对Ni(OH)2超级电容器材料的电化学行为的影响[J]. 电化学, 2011 , 17(3) : 292 -299 . DOI: 10.61558/2993-074X.2843
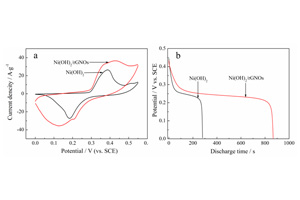
Key words: graphene; Ni(OH)2 nanoparticle; electrochemical deposition; supercapacitor
/
| 〈 |
|
〉 |