

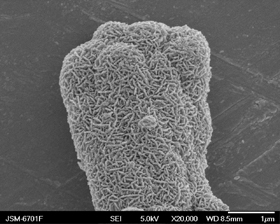
介孔碳基钴镍氧化物的电化学电容性能
收稿日期: 2011-01-07
修回日期: 2011-03-15
网络出版日期: 2011-05-05
基金资助
国家自然科学基金(50602020), 973计划前期研究专项(2007CB216408)和甘肃省高校基本科研业务费专项资金
Electrochemical Capacitive Performance of Mesoporous Carbon Based Co-Ni Oxides
Received date: 2011-01-07
Revised date: 2011-03-15
Online published: 2011-05-05
杨贞胜 , 孔令斌 , 邓莉 , 罗永春 , 康龙 . 介孔碳基钴镍氧化物的电化学电容性能[J]. 电化学, 2011 , 17(2) : 217 -221 . DOI: 10.61558/2993-074X.2834

Key words: supercapacitor; ; cobalt-nickel oxide; ; interconnected nanoflakes network
/
| 〈 |
|
〉 |