

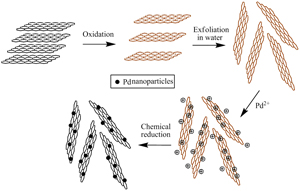
不同载体负载Pd催化剂对甲酸的电催化氧化活性比较
收稿日期: 2010-10-08
修回日期: 2010-12-15
网络出版日期: 2011-05-05
基金资助
国家自然科学基金(50701023)以及江苏省博士创新基金(CX09B_075Z)
Comparison of Catalytic Performance on Different Materials Supported Pd Catalysts for Formic Acid Oxidation
Received date: 2010-10-08
Revised date: 2010-12-15
Online published: 2011-05-05
杨苏东 , 梁彦瑜 , 温祝亮 , 宋启军 , 张校刚 . 不同载体负载Pd催化剂对甲酸的电催化氧化活性比较[J]. 电化学, 2011 , 17(2) : 175 -179 . DOI: 10.61558/2993-074X.2831
Key words: Palladium; Formic acid oxidation; Supports; Catalyst
/
| 〈 |
|
〉 |